Drug Information
Drug (ID: DG01584) and It's Reported Resistant Information
| Name |
LY-2874455
|
||||
|---|---|---|---|---|---|
| Synonyms |
LY2874455; 1254473-64-7; LY-2874455; UNII-E9M363811V; LY 2874455; CHEMBL3828009; E9M363811V; (R,E)-2-(4-(2-(5-(1-(3,5-dichloropyridin-4-yl)ethoxy)-1H-indazol-3-yl)vinyl)-1H-pyrazol-1-yl)ethanol; C21H19Cl2N5O2; (R,E)-2-(4-(2-(5-(1-(3,5-dichloropyridin-4-yl)ethoxy)-1H-indazol-3-yl)vinyl)-1H-pyrazol-1-yl)ethanol.; 2-[4-[(E)-2-[5-[(1R)-1-(3,5-dichloropyridin-4-yl)ethoxy]-1H-indazol-3-yl]ethenyl]pyrazol-1-yl]ethanol; (R,E)-2-(4-(2-(5-(1-(3,5-Dichloropyridin-4-yl)-ethoxy)-1H-indazol-3-yl)vinyl)-1H-pyrazol-1-yl)ethanol; LY2874455 monohydrate; SCHEMBL298445; SCHEMBL298446; GTPL8104; QCR-90; DTXSID20154776; BCP04756; EX-A1340; 2529AH; BDBM50189781; NSC771750; NSC783670; s7057; ZINC73069242; AKOS027251051; BCP9000878; CCG-269169; CS-0907; DB13022; LM 1476; NSC-771750; NSC-783670; (R,E)-2-(4-(2-(5-(1-(3,5-dichloropyridin-4-yl)ethoxy)-1H-indazol-3-yl)vinyl)-1H-pyrazol-1-yl)ethan-1-ol; AC-33003; AS-74798; HY-13304; BCP0726000015; C72002; LY2874455/LY-2874455; J-690104; Q27082753; (R)-(E)-2-(4-(2-(5-(1-(3,5-Dichloropyridin-4-yl)ethoxy)-1H-indazol-3-yl)vinyl)-1H-pyrazol-1-yl)ethanol; 2-{4-[(1E)-2-{5-[(1R)-1-(3,5-dichloropyridin-4-yl)ethoxy]-1H-indazol-3-yl}ethenyl]-1H-pyrazol-1-yl}ethan-1-ol
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
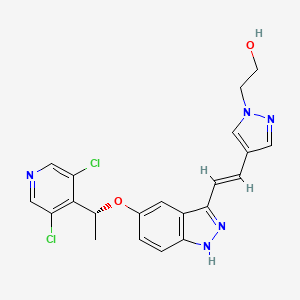
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Fibroblast growth factor receptor 2 (FGFR2) | FGFR2_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
7
|
||||
| IsoSMILES |
C[C@H](C1=C(C=NC=C1Cl)Cl)OC2=CC3=C(C=C2)NN=C3/C=C/C4=CN(N=C4)CCO
|
||||
| InChI |
InChI=1S/C21H19Cl2N5O2/c1-13(21-17(22)10-24-11-18(21)23)30-15-3-5-20-16(8-15)19(26-27-20)4-2-14-9-25-28(12-14)6-7-29/h2-5,8-13,29H,6-7H2,1H3,(H,26,27)/b4-2+/t13-/m1/s1
|
||||
| InChIKey |
GKJCVYLDJWTWQU-CXLRFSCWSA-N
|
||||
| PubChem CID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Fibroblast growth factor receptor 3 (FGFR3) | [1] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.N540K (c.1620C>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Gallbladder | N.A. | ||
| Experiment for Molecule Alteration |
Targeted sequencing of tumor tissue assay | |||
| Mechanism Description | The missense mutation p.N540K (c.1620C>G) in gene FGFR3 cause the resistance of LY-2874455 by aberration of the drug's therapeutic target | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Fibroblast growth factor receptor 3 (FGFR3) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.K650E (c.1948A>G) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.53 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.34 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
M
-
G
-
S
-
S
-
H
440
|
-
H
-
H
-
H
-
H
-
H
-
S
-
Q
G
D
S
P
H
P
450
|
M
T
L
L
A
A
G
N
V
V
S
S
E
E
Y
L
E
E
L
L
460
|
P
P
E
A
D
D
P
P
K
K
W
W
E
E
F
L
P
S
R
R
470
|
D
A
K
R
L
L
T
T
L
L
G
G
K
K
P
P
L
L
G
G
480
|
E
E
G
G
C
A
F
F
G
G
Q
Q
V
V
V
V
M
M
A
A
490
|
E
E
A
A
I
I
G
G
I
I
D
D
K
K
D
D
R
R
A
A
500
|
A
A
K
K
P
P
V
V
T
T
V
V
A
A
V
V
K
K
M
M
510
|
L
L
K
K
D
D
D
D
A
A
T
T
D
D
K
K
D
D
L
L
520
|
S
S
D
D
L
L
V
V
S
S
E
E
M
M
E
E
M
M
M
M
530
|
K
K
M
M
I
I
G
G
K
K
H
H
K
K
N
N
I
I
I
I
540
|
N
N
L
L
L
L
G
G
A
A
C
C
T
T
Q
Q
G
G
G
G
550
|
P
P
L
L
Y
Y
V
V
L
L
V
V
E
E
Y
Y
A
A
A
A
560
|
K
K
G
G
N
N
L
L
R
R
E
E
F
F
L
L
R
R
A
A
570
|
R
R
R
R
P
P
P
P
G
G
L
L
D
D
Y
Y
S
S
F
F
580
|
D
D
T
T
C
S
K
K
P
P
P
P
E
E
E
E
Q
Q
L
L
590
|
T
T
F
F
K
K
D
D
L
L
V
V
S
S
C
C
A
A
Y
Y
600
|
Q
Q
V
V
A
A
R
R
G
G
M
M
E
E
Y
Y
L
L
A
A
610
|
S
S
Q
Q
K
K
C
C
I
I
H
H
R
R
D
D
L
L
A
A
620
|
A
A
R
R
N
N
V
V
L
L
V
V
T
T
E
E
D
D
N
N
630
|
V
V
M
M
K
K
I
I
A
A
D
D
F
F
G
G
L
L
A
A
640
|
R
R
D
D
V
V
H
H
N
N
L
L
D
D
Y
Y
Y
Y
K
K
650
|
K
E
T
T
T
T
N
N
G
G
R
R
L
L
P
P
V
V
K
K
660
|
W
W
M
M
A
A
P
P
E
E
A
A
L
L
F
F
D
D
R
R
670
|
V
V
Y
Y
T
T
H
H
Q
Q
S
S
D
D
V
V
W
W
S
S
680
|
F
F
G
G
V
V
L
L
L
L
W
W
E
E
I
I
F
F
T
T
690
|
L
L
G
G
G
G
S
S
P
P
Y
Y
P
P
G
G
I
I
P
P
700
|
V
V
E
E
E
E
L
L
F
F
K
K
L
L
L
L
K
K
E
E
710
|
G
G
H
H
R
R
M
M
D
D
K
K
P
P
A
A
N
N
C
C
720
|
T
T
H
H
D
D
L
L
Y
Y
M
M
I
I
M
M
R
R
E
E
730
|
C
C
W
W
H
H
A
A
A
A
P
P
S
S
Q
Q
R
R
P
P
740
|
T
T
F
F
K
K
Q
Q
L
L
V
V
E
E
D
D
L
L
D
D
750
|
R
R
V
V
L
L
T
T
V
V
T
T
S
S
T
T
D
D
E
E
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Gallbladder | N.A. | |||||||||||
| Experiment for Molecule Alteration |
Targeted sequencing of tumor tissue assay | ||||||||||||
| Mechanism Description | The missense mutation p.K650E (c.1948A>G) in gene FGFR3 cause the sensitivity of LY-2874455 by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: Fibroblast growth factor receptor 3 (FGFR3) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V555M (c.1663G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.53 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.35 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
G
-
S
-
H
-
450
|
M
-
L
-
A
-
G
-
V
M
S
S
E
E
Y
L
E
E
L
L
460
|
P
P
E
A
D
D
P
P
K
K
W
W
E
E
F
L
P
S
R
R
470
|
D
A
K
R
L
L
T
T
L
L
G
G
K
K
P
P
L
L
G
G
480
|
E
E
G
G
C
C
F
F
G
G
Q
Q
V
V
V
V
M
M
A
A
490
|
E
E
A
A
I
I
G
G
I
I
D
D
K
K
D
D
R
R
A
A
500
|
A
A
K
K
P
P
V
V
T
T
V
V
A
A
V
V
K
K
M
M
510
|
L
L
K
K
D
D
D
D
A
A
T
T
D
D
K
K
D
D
L
L
520
|
S
S
D
D
L
L
V
V
S
S
E
E
M
M
E
E
M
M
M
M
530
|
K
K
M
M
I
I
G
G
K
K
H
H
K
K
N
N
I
I
I
I
540
|
N
N
L
L
L
L
G
G
A
A
C
C
T
T
Q
Q
G
G
G
G
550
|
P
P
L
L
Y
Y
V
V
L
L
V
M
E
E
Y
Y
A
A
A
A
560
|
K
K
G
G
N
N
L
L
R
R
E
E
F
F
L
L
R
R
A
A
570
|
R
R
R
R
P
S
P
G
G
-
L
-
D
-
Y
-
S
-
F
-
580
|
D
-
T
-
C
-
K
-
P
-
P
-
E
E
E
E
Q
Q
L
L
590
|
T
T
F
F
K
K
D
D
L
L
V
V
S
S
C
C
A
A
Y
Y
600
|
Q
Q
V
V
A
A
R
R
G
G
M
M
E
E
Y
Y
L
L
A
A
610
|
S
S
Q
Q
K
K
C
C
I
I
H
H
R
R
D
D
L
L
A
A
620
|
A
A
R
R
N
N
V
V
L
L
V
V
T
T
E
E
D
D
N
N
630
|
V
V
M
M
K
K
I
I
A
A
D
D
F
F
G
G
L
L
A
A
640
|
R
R
D
D
V
V
H
H
N
N
L
L
D
D
Y
Y
Y
Y
K
K
650
|
K
K
T
T
T
T
N
N
G
G
R
R
L
L
P
P
V
V
K
K
660
|
W
W
M
M
A
A
P
P
E
E
A
A
L
L
F
F
D
D
R
R
670
|
V
V
Y
Y
T
T
H
H
Q
Q
S
S
D
D
V
V
W
W
S
S
680
|
F
F
G
G
V
V
L
L
L
L
W
W
E
E
I
I
F
F
T
T
690
|
L
L
G
G
G
G
S
S
P
P
Y
Y
P
P
G
G
I
I
P
P
700
|
V
V
E
E
E
E
L
L
F
F
K
K
L
L
L
L
K
K
E
E
710
|
G
G
H
H
R
R
M
M
D
D
K
K
P
P
A
A
N
N
C
C
720
|
T
T
H
H
D
D
L
L
Y
Y
M
M
I
I
M
M
R
R
E
E
730
|
C
C
W
W
H
H
A
A
A
A
P
P
S
S
Q
Q
R
R
P
P
740
|
T
T
F
F
K
K
Q
Q
L
L
V
V
E
E
D
D
L
L
D
D
750
|
R
R
V
V
L
L
T
T
V
V
T
T
S
S
T
H
D
H
E
H
760
|
-
H
-
H
-
H
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Gallbladder | N.A. | |||||||||||
| Experiment for Molecule Alteration |
Targeted sequencing of tumor tissue assay | ||||||||||||
| Mechanism Description | The missense mutation p.V555M (c.1663G>A) in gene FGFR3 cause the sensitivity of LY-2874455 by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: Fibroblast growth factor receptor 3 (FGFR3) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.L608V (c.1822T>G) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Gallbladder | N.A. | |||||||||||
| Experiment for Molecule Alteration |
Targeted sequencing of tumor tissue assay | ||||||||||||
| Mechanism Description | The missense mutation p.L608V (c.1822T>G) in gene FGFR3 cause the sensitivity of LY-2874455 by aberration of the drug's therapeutic target | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
