Drug Information
Drug (ID: DG01546) and It's Reported Resistant Information
| Name |
Patidegib
|
||||
|---|---|---|---|---|---|
| Synonyms |
SARIDEGIB; Patidegib; IPI-926; 1037210-93-7; IPI 926; UNII-JT96FPU35X; JT96FPU35X; CHEMBL538867; N-((2S,3R,3aS,3'R,4a'R,6S,6a'R,6b'S,7aR,12a'S,12b'S)-3,6,11',12b'-tetramethyl-2',3a,3',4,4',4a',5,5',6,6',6a',6b',7,7a,7',8',10',12',12a',12b'-icosahydro-1'H,3H-spiro[furo[3,2-b]pyridine-2,9'-naphtho[2,1-a]azulen]-3'-yl)methanesulfonamide; N-[(3R,3'R,3'aS,4aR,6'S,6aR,6bS,7'aR,9S,12aS,12bS)-3',6',11,12b-tetramethylspiro[1,2,3,4,4a,5,6,6a,6b,7,8,10,12,12a-tetradecahydronaphtho[2,1-a]azulene-9,2'-3a,4,5,6,7,7a-hexahydro-3H-furo[3,2-b]pyridine]-3-yl]methanesulfonamide; Patidegib [USAN:INN]; Saridegib [Rescinded USAN]; Patidegib (USAN); IP9 FREE BASE; FIN-5; IPI-926 FREE BASE; SCHEMBL421999; GTPL8198; IP-9; DTXSID40146032; CHEBI:177425; Saridegib (IPI-926; Patidegib); WHO 9619; BDBM50293788; ZINC43197072; DB12655; HY-16587; CS-0007501; D10324; Q15426668; Methanesulfonamide, N-((2S,3R,3'R,3aS,4'aR,6S,6'aR,6'bS,7aR,12'aS,12'bS)- 2',3',3a,4,4',4'a,5,5',6,6',6'a,6'b,7,7',7a,8',10',12',12'a,12'b-eicosahydro-3,6,11',12'b-tetramethylspiro(furo(3,2-b)pyridine-2(3H),9'(1'H)-naphth(2,1-a)azulen)-3'-yl)-; N-((2S,3R,3aS,3''R,4a''R,6S,6a''R,6b''S,7aR,12a''S,12b''S)-3,6,11'',12b''-tetramethyl-2'',3a,3'',4,4'',4a'',5,5'',6,6'',6a'',6b'',7,7a,7'',8'',10'',12'',12a'',12b''-icosahydro-1''H,3H-spiro[furo[3,2-b]pyridine-2,9''-naphtho[2,1-a]azulene]-3''-yl)methanesulfonamide; N-[(3R,3'R,3'aS,4aR,6'S,6aR,6bS,7'aR,9S,12aS,12bS)-3',6',11,12b-tetramethylspiro[1,2,3,4,4a,5,6,6a,6b,7,8,10,12,12a-tetradecahydronaphtho[2,1-a]azulene-9,2'-3a,4,5,6,7,7a-hexahydro-3H-uro[3,2-b]pyridine]-3-yl]methanesulonamide
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
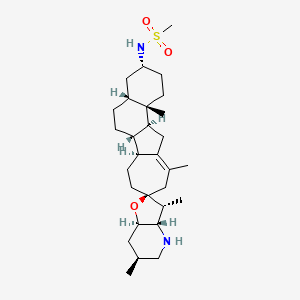
|
||||
| Target | E2 ubiquitin-conjugating enzyme T (UBE2T) | UBE2T_HUMAN | [1] | ||
| RAC-gamma serine/threonine-protein kinase (AKT3) | AKT3_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
2
|
||||
| IsoSMILES |
C[C@H]1C[C@@H]2[C@H]([C@H]([C@]3(O2)CC[C@H]4[C@@H]5CC[C@@H]6C[C@@H](CC[C@@]6([C@H]5CC4=C(C3)C)C)NS(=O)(=O)C)C)NC1
|
||||
| InChI |
InChI=1S/C29H48N2O3S/c1-17-12-26-27(30-16-17)19(3)29(34-26)11-9-22-23-7-6-20-13-21(31-35(5,32)33)8-10-28(20,4)25(23)14-24(22)18(2)15-29/h17,19-23,25-27,30-31H,6-16H2,1-5H3/t17-,19+,20+,21+,22-,23-,25-,26+,27-,28-,29-/m0/s1
|
||||
| InChIKey |
HZLFFNCLTRVYJG-WWGOJCOQSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Smoothened homolog (SMO) | [1] | |||
| Sensitive Disease | Medulloblastoma [ICD-11: 2A00.10] | |||
| Molecule Alteration | Missense mutation | p.D473H (c.1417G>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Bone marrow | N.A. | ||
| In Vivo Model | Mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Mechanism Description | The missense mutation p.D473H (c.1417G>C) in gene SMO cause the sensitivity of Patidegib by unusual activation of pro-survival pathway | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
