Drug Information
Drug (ID: DG01501) and It's Reported Resistant Information
| Name |
Tofacitinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Tofacitinib; Tasocitinib; 477600-75-2; 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile; CP-690550; CP 690550; 1259404-17-5; rac-Tofacitinib; CP-690,550; UNII-87LA6FU830; Tofacitinib (CP-690550,Tasocitinib); 3-((3R,4R)-rel-4-Methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile; racemic-tofacitinib; 3-{(3R,4R)-4-methyl-3-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl}-3-oxopropanenitrile; CP690550; CHEMBL221959; CHEBI:71200; 87LA6FU830; 477600-75-2 (free base); MFCD11035919; 3-((3R,4R)-4-Methyl-3-(methyl(7H-pyrrolo(2,3-d)pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile; 3-[(3R*,4R*)-4-Methyl-3-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl]-3-oxopropanenitrile; 3-[(3R,4R)-4-methyl-3-[methyl({7H-pyrrolo[2,3-d]pyrimidin-4-yl})amino]piperidin-1-yl]-3-oxopropanenitrile; 3-[(3R,4R)-4-methyl-3-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl]-3-oxopropanenitrile; 3-{(3R,4R)-4-Methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3-oxo-propionitrile; Tofacitinib [USAN:INN]; C16H20N6O; tofacitinibum; 3eyg; 3fup; 3lxk; 4oti; MI1; Tofacitinib (USAN); Cp-690 free base; 1-Piperidinepropanenitrile, 4-methyl-3-(methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-beta-oxo-, (3R,4R)-; SCHEMBL322753; Tofacitinib (CP-690550); GTPL5677; QCR-53; HSDB 8311; AMY3992; DTXSID90197271; EX-A205; BCPP000274; CP-690550 FREE BASE; AOB87470; ZINC3818808; CP-690,550 FREE BASE; BDBM50193995; CP-690; NSC782351; NSC800953; s2789; AKOS005145814; AKOS005258733; AC-8193; BCP9000550; CA10005; CCG-264998; CS-0050; DB08895; GS-6106; NSC-782351; NSC-800953; NCGC00229511-02; NCGC00229511-04; NCGC00229511-10; NCGC00244463-01; Tofacitinib (CP-690550, Tasocitinib); HY-40354; CP690,550; CP-690-550; W6440; CP- 690 550; D09970; AB01565786_02; 600M752; AR-270/43507983; J-524314; Q3530324; BRD-K31283835-001-01-9; BRD-K31283835-048-04-4; 3-((3R,4R)-4-Methyl(7H-Pyrrolo[2,3-D]Pyrimidin-4-Yi)Amino)Piperidin-1-Yl-3-Oxopropaneni; (3R,4R)-3-{4-Methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]-piperidin-1-yl}-3-oxo-propionitrile; (3R,4R)-4-methyl-3-(methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-beta-oxo-1-piperidine propanenitrile; 3-[(3R,4R)-4-methyl-3-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]-1-piperidyl]-3-oxo-propanenitrile; 3-{(3R,4R)-4-methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amino]piperidin-1-yl}-3-oxo-propionitrile; 3-{(3R,4R)-4-Methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin4-yl)-amino]-piperidin-1-yl}-3-oxo-propionitrile; 3-{4-methyl-3-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]-1-piperidinyl}-3-oxopropanenitrile; 3-Piperidinamine, 1-(cyanoacetyl)-4-methyl-N-methyl-N-1H-pyrrolo(2,3-d)pyrimidin-4-yl-, (3R,4R)-; Tofacitinib;3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
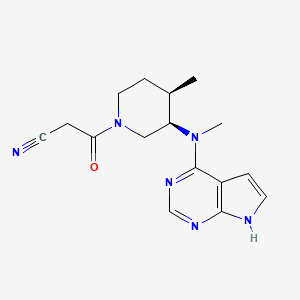
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
|
||||
| Target | Janus kinase 3 (JAK-3) | JAK3_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
3
|
||||
| IsoSMILES |
C[C@@H]1CCN(C[C@@H]1N(C)C2=NC=NC3=C2C=CN3)C(=O)CC#N
|
||||
| InChI |
InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1
|
||||
| InChIKey |
UJLAWZDWDVHWOW-YPMHNXCESA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.L857P (c.2570T>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| U4C cells | Acetabulum | Homo sapiens (Human) | CVCL_D314 | |
| In Vivo Model | Balb/c bone marrow transplantation mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Proliferation assay | |||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [3] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.E183G (c.548A>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.E183G (c.548A>G) in gene JAK3 cause the sensitivity of Tofacitinib by aberration of the drug's therapeutic target | |||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.V674A (c.2021T>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| U4C cells | Acetabulum | Homo sapiens (Human) | CVCL_D314 | |
| In Vivo Model | Balb/c bone marrow transplantation mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Proliferation assay | |||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [3] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.A572V (c.1715C>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.A572V (c.1715C>T) in gene JAK3 cause the sensitivity of Tofacitinib by aberration of the drug's therapeutic target | |||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [3] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.L156P (c.467T>C) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.L156P (c.467T>C) in gene JAK3 cause the sensitivity of Tofacitinib by aberration of the drug's therapeutic target | |||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [3] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.R172Q (c.515G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | The missense mutation p.R172Q (c.515G>A) in gene JAK3 cause the sensitivity of Tofacitinib by aberration of the drug's therapeutic target | |||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [4] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.V722I (c.2164G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | K562 cells | Blood | Homo sapiens (Human) | CVCL_0004 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Trypan blue exclusion assay | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: CAMPATH-1 antigen (CD52) | [2] | |||
| Resistant Disease | t-cell prolymphocytic leukemia [ICD-11: 2A90.0] | |||
| Molecule Alteration | Expressiom | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | T-cell prolymphocytic leukemia patient | Homo sapiens | ||
| Experiment for Molecule Alteration |
Flow cytometry | |||
| Experiment for Drug Resistance |
Overall survival assay | |||
| Mechanism Description | MTX-HOPE is a combination of classical chemotherapy agents originally developed for palliative chemotherapy in frail patients with refractory lymphoma. MTX-HOPE has been reported to be effective against T-cell tumors. Severe nonhematologic adverse events are rarely reported; however, bone marrow suppression is commonly observed. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [5] | |||
| Sensitive Disease | Mature T-cell and Nk-cell lymphoma [ICD-11: 2B0Y.0] | |||
| Molecule Alteration | Missense mutation | p.A572V (c.1715C>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NK-S1 cells | N.A. | N.A. | N.A. |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.A572V (c.1715C>T) in gene JAK3 cause the sensitivity of Tofacitinib by aberration of the drug's therapeutic target | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [6] | |||
| Sensitive Disease | Hematologic Cancer [ICD-11: MG24.Y] | |||
| Molecule Alteration | Missense mutation | p.M511I (c.1533G>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; FACS assay | |||
| Mechanism Description | Jak3 inhibitors CP-690,550 and NC1153 showed efficacy in reducing viability of Ba/F3 cells transformed with mutant forms of Jak3. | |||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [6] | |||
| Sensitive Disease | Hematologic Cancer [ICD-11: MG24.Y] | |||
| Molecule Alteration | Missense mutation | p.A573V (c.1718C>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay; FACS assay | |||
| Mechanism Description | Jak3 inhibitors CP-690,550 and NC1153 showed efficacy in reducing viability of Ba/F3 cells transformed with mutant forms of Jak3. | |||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [7] | |||
| Sensitive Disease | Hematologic Cancer [ICD-11: MG24.Y] | |||
| Molecule Alteration | Missense mutation | p.H583Y (c.1747C>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | JAKT/STAT signaling pathway | Inhibition | hsa04630 | |
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| NIH-3T3 cells | Embryo | Mus musculus (Mouse) | CVCL_0594 | |
| Experiment for Molecule Alteration |
Immunohistochemistry assay; Immunoblotting assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Mechanism Description | A STAT3 inhibitor was active against STAT3 -mutant SNK-6 and YT cells. Novel JAK3 mutations are oncogenic and druggable in NTCL. The JAK3 or STAT3 signal was altered in NTCL, and pathway inhibition might be a therapeutic option for patients with JAK3 - or STAT3 -mutant NTCL. | |||
| Key Molecule: Tyrosine-protein kinase JAK3 (JAK3) | [7] | |||
| Sensitive Disease | Hematologic Cancer [ICD-11: MG24.Y] | |||
| Molecule Alteration | Missense mutation | p.G589D (c.1766G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | JAKT/STAT signaling pathway | Inhibition | hsa04630 | |
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| NIH-3T3 cells | Embryo | Mus musculus (Mouse) | CVCL_0594 | |
| Experiment for Molecule Alteration |
Immunohistochemistry assay; Immunoblotting assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Mechanism Description | A STAT3 inhibitor was active against STAT3 -mutant SNK-6 and YT cells. Novel JAK3 mutations are oncogenic and druggable in NTCL. The JAK3 or STAT3 signal was altered in NTCL, and pathway inhibition might be a therapeutic option for patients with JAK3 - or STAT3 -mutant NTCL. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
