Drug Information
Drug (ID: DG01495) and It's Reported Resistant Information
| Name |
SU5614
|
||||
|---|---|---|---|---|---|
| Synonyms |
SU-5614; 1055412-47-9; su5614; (Z)-5-Chloro-3-((3,5-dimethyl-1H-pyrrol-2-yl)methylene)indolin-2-one; CHEMBL2062155; CHEBI:87159; 186611-56-3; SU 5614; (Z)-SU5614; AC1NS4RE; (3Z)-5-chloro-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-1H-indol-2-one; (3Z)-5-Chloro-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylene]-1,3-dihydro-2H-indol-2-one; MFCD08443928; 5-Chloro-3-[(3,5-dimethylpyrrol-2-yl)methylene]-2-indolinone; 5-CHLORO-3-(3,5-DIMETHYL-1H-PYRROL-2-YLMETHYLENE)-1,3-DIHYDRO-INDOL-2-ONE; EX-A821; ZINC3875026; BDBM50415432; HY-18952A; AKOS030527565; CID:6536806; NCGC00480782-01; AS-70968; DS-019384; SU5614, >=98% (HPLC); CS-0029269; A895990; J-690017; Q27159416; 5-chloro-3-((3,5-dimethyl-1H-pyrrol-2-yl)methylene)indolin-2-one; 5-chloro-3-(3,5-dimethyl-1h-pyrrol-2-ylmethylene)-1,3-dihydroindol-2-one; (3Z)-5-chloro-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-1,3-dihydro-2H-indol-2-one; (3Z)-5-chloro-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-2,3-dihydro-1H-indol-2-one; 3-Chloro-N-[(1S)-2-[[2-(dimethylamino)acetyl]amino]-1-[[4-[8-[(1S)-1-hydroxyethyl]imidazo[1,2-a]pyridin-2-yl]phenyl]methyl]ethyl]-4- (1-methylethoxy)-benzamide
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
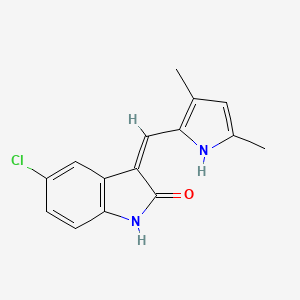
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[2]
|
||||
| Target | Heat shock protein 90 alpha (HSP90A) | HS90A_HUMAN | [2] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
1
|
||||
| IsoSMILES |
CC1=CC(=C(N1)/C=C\\2/C3=C(C=CC(=C3)Cl)NC2=O)C
|
||||
| InChI |
InChI=1S/C15H13ClN2O/c1-8-5-9(2)17-14(8)7-12-11-6-10(16)3-4-13(11)18-15(12)19/h3-7,17H,1-2H3,(H,18,19)/b12-7-
|
||||
| InChIKey |
XLBQNZICMYZIQT-GHXNOFRVSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [2] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | IF-deletion | p.Q569_G613 (c.1705_1837) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The if-deletion p.Q569_G613 (c.1705_1837) in gene FLT3 cause the resistance of SU5614 by unusual activation of pro-survival pathway. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [1] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.D835Y (c.2503G>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Mechanism Description | The missense mutation p.D835Y (c.2503G>T) in gene FLT3 cause the resistance of SU5614 by unusual activation of pro-survival pathway | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
