Drug Information
Drug (ID: DG01472) and It's Reported Resistant Information
| Name |
Staurosporine
|
||||
|---|---|---|---|---|---|
| Synonyms |
Staurosporine; Staurosporin; 62996-74-1; (+)-Staurosporine; Antibiotic 230; Antibiotic AM 2282; AM-2282; CCRIS 3272; Antibiotic AM-2282; UNII-H88EPA0A3N; Alkaloid AM-2282 from Streptomyces; H88EPA0A3N; CHEMBL388978; CHEBI:15738; 8,12-Epoxy-1H,8H-2,7b,12a-triazadibenzo(a,g)cyclonona(cde)trinden-1-one, 2,3,9,10,11,12-hexahydro-9-methoxy-8-methyl-10-(methylamino)-, (8alpha,9beta,10beta,12alpha)-(+)-; (5S,6R,7R,9R)-6-methoxy-5-methyl-7-(methylamino)-6,7,8,9,15,16-hexahydro-5H,14H-17-oxa-4b,9a,15-triaza-5,9-methanodibenzo[b,h]cyclonona[jkl]cyclopenta[e]-as-indacen-14-one; (5S,6R,7R,9R)-6-methoxy-5-methyl-7-methylamino-6,7,8,9,15,16-hexahydro-5H,14H-5,9-epoxy-4b,9a,15-triazadibenzo[b,h]cyclonona[1,2,3,4-jkl]cyclopenta[e]-as-indacen-14-one; GNF-PF-1389; methoxy-methyl-(methylamino)[ ]one; SR-00000001485; Staurosporin, 4; 1nvr; 1stc; 1xbc; 1xjd; 1yhs; 2gcd; Staurosporine, 8; CGP 39360; Staurosporine & TNF; 1q3d; 1sm2; 2dq7; MolMap_000047; SCHEMBL8157; CBiol_001978; BSPBio_001146; GTPL346; BDBM2579; MEGxm0_000307; DTXSID6041131; 1u59; BCPP000063; Bio1_000264; Bio1_000753; Bio1_001242; HMS1990J07; HMS3650B17; EX-A1777; ZINC3814434; AM2282; NSC755774; s1421; AKOS015897119; AM 2282; CCG-208052; DB02010; NSC-755774; QTL1_000078; NCGC00162400-01; NCGC00162400-02; NCGC00162400-03; NCGC00162400-04; NCGC00162400-05; NCGC00162400-06; NCGC00162400-09; 9,13-Epoxy-1H,9H-diindolo(1,2,3-gh:3',2',1'-lm)pyrrolo(3,4-j)(1,7)-benzodiazonin-1-one, 2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-, (9S-(9alpha,10beta,11beta,13alpha)-; HY-15141; Staurosporine & Tumor necrosis factor (TNF); Staurosporine 100 microg/mL in Acetonitrile; Q5957181; SR-00000001485-4; BRD-K17953061-001-02-8; BRD-K17953061-001-04-4; BRD-K17953061-001-05-1; BRD-K17953061-001-08-5; BRD-K17953061-001-10-1; BRD-K17953061-001-11-9; (2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-29-oxa-1,7,17-triazaoctacyclo[12.12.2.1^{2,6}.0^{7,28}.0^{8,13}.0^{15,19}.0^{20,27}.0^{21,26}]nonacosa-8(13),9,11,14(28),15(19),20(27),21(26),22,24-nonaen-16-one; (2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-29-oxa-1,7,17-triazaoctacyclo[12.12.2.1^{2,6}.0^{7,28}.0^{8,13}.0^{15,19}.0^{20,27}.0^{21,26}]nonacosa-8,10,12,14(28),15(19),20(27),21,23,25-nonaen-16-one; (2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-29-oxa-1,7,17-triazaoctacyclo[12.12.2.12,6.07,28.08,13.015,19.020,27.021,26]nonacosa-8,10,12,14,19,21,23,25,27-nonaen-16-one; (5S,6R,7R,9R)-6-methoxy-5-methyl-7-(methylamino)-6,7,8,9,15,16-hexahydro-5H,14H-5,9-epoxy-4b,9a,15-triazadibenzo[b,h]cyclonona[1,2,3,4-jkl]cyclopenta[e]-as-indacen-14-one; [9S-(9 ,10 ,11 ,13 )]-2,3,10,11,12,13-Hexahydro-10-methoxy-9-methyl-11-(methylamino)-9,13-epoxy-1H,9H-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one; 109189-95-9; 9,13-Epoxy-1H,9H-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one, 2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-, (9S,10R,11R,13R)- & Tumor necrosis factor (TNF); 9,13-Epoxy-1H,9H-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one, 2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-, (9S,10R,11R,13R)- (9CI); 9,13-Epoxy-1H,9H-diindolo[1,2,3-gh:3',2',1'-lm]pyrrolo[3,4-j][1,7]benzodiazonin-1-one, 2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-, [9S-(9alpha,10beta,11beta,13alpha)]-
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
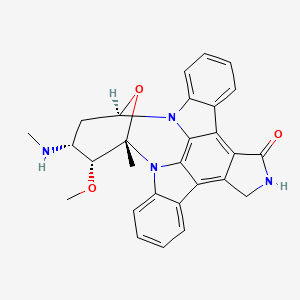
|
||||
| Target | Bacterial Penicillin binding protein (Bact PBP) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
2
|
||||
| IsoSMILES |
C[C@@]12[C@@H]([C@@H](C[C@@H](O1)N3C4=CC=CC=C4C5=C6C(=C7C8=CC=CC=C8N2C7=C53)CNC6=O)NC)OC
|
||||
| InChI |
InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1
|
||||
| InChIKey |
HKSZLNNOFSGOKW-FYTWVXJKSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Epidermal growth factor receptor (EGFR) | [1] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.T790M (c.2369C>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 3.10 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 3.05 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
S
G
G
E
E
A
A
P
P
700
|
N
N
Q
Q
A
A
L
L
L
L
R
R
I
I
L
L
K
K
E
E
710
|
T
T
E
E
F
F
K
K
K
K
I
I
K
K
V
V
L
L
G
G
720
|
S
S
G
G
A
A
F
F
G
G
T
T
V
V
Y
Y
K
K
G
G
730
|
L
L
W
W
I
I
P
P
E
E
G
G
E
E
K
K
V
V
K
K
740
|
I
I
P
P
V
V
A
A
I
I
K
K
E
E
L
L
R
R
E
E
750
|
A
A
T
T
S
S
P
P
K
K
A
A
N
N
K
K
E
E
I
I
760
|
L
L
D
D
E
E
A
A
Y
Y
V
V
M
M
A
A
S
S
V
V
770
|
D
D
N
N
P
P
H
H
V
V
C
C
R
R
L
L
L
L
G
G
780
|
I
I
C
C
L
L
T
T
S
S
T
T
V
V
Q
Q
L
L
I
I
790
|
T
M
Q
Q
L
L
M
M
P
P
F
F
G
G
C
C
L
L
L
L
800
|
D
D
Y
Y
V
V
R
R
E
E
H
H
K
K
D
D
N
N
I
I
810
|
G
G
S
S
Q
Q
Y
Y
L
L
L
L
N
N
W
W
C
C
V
V
820
|
Q
Q
I
I
A
A
K
K
G
G
M
M
N
N
Y
Y
L
L
E
E
830
|
D
D
R
R
R
R
L
L
V
V
H
H
R
R
D
D
L
L
A
A
840
|
A
A
R
R
N
N
V
V
L
L
V
V
K
K
T
T
P
P
Q
Q
850
|
H
H
V
V
K
K
I
I
T
T
D
D
F
F
G
G
L
L
A
A
860
|
K
K
L
L
L
L
G
G
A
A
E
E
E
E
K
K
E
E
Y
Y
870
|
H
H
A
A
E
E
G
G
G
G
K
K
V
V
P
P
I
I
K
K
880
|
W
W
M
M
A
A
L
L
E
E
S
S
I
I
L
L
H
H
R
R
890
|
I
I
Y
Y
T
T
H
H
Q
Q
S
S
D
D
V
V
W
W
S
S
900
|
Y
Y
G
G
V
V
T
T
V
V
W
W
E
E
L
L
M
M
T
T
910
|
F
F
G
G
S
S
K
K
P
P
Y
Y
D
D
G
G
I
I
P
P
920
|
A
A
S
S
E
E
I
I
S
S
S
S
I
I
L
L
E
E
K
K
930
|
G
G
E
E
R
R
L
L
P
P
Q
Q
P
P
P
P
I
I
C
C
940
|
T
T
I
I
D
D
V
V
Y
Y
M
M
I
I
M
M
V
V
K
K
950
|
C
C
W
W
M
M
I
I
D
D
A
A
D
D
S
S
R
R
P
P
960
|
K
K
F
F
R
R
E
E
L
L
I
I
I
I
E
E
F
F
S
S
970
|
K
K
M
M
A
A
R
R
D
D
P
P
Q
Q
R
R
Y
Y
L
L
980
|
V
V
I
I
Q
Q
G
G
D
D
E
E
R
R
M
M
H
H
L
L
990
|
P
P
S
S
P
P
T
T
D
D
S
S
N
N
F
F
Y
Y
R
R
1000
|
A
A
L
L
M
M
D
D
E
E
E
E
D
D
M
M
D
D
D
D
1010
|
V
V
V
V
D
D
A
A
D
D
E
E
Y
Y
L
L
I
I
P
P
1020
|
Q
Q
Q
Q
G
G
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | Lung | N.A. | |||||||||||
ICD-X: Extension Codes
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Serine/threonine-protein kinase (SSTK) | [2] | |||
| Sensitive Disease | s. suis infection [ICD-11: XN5SE] | |||
| Molecule Alteration | Phosphorylation | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Streptococcus suis SC19 | 5833 | ||
| Experiment for Molecule Alteration |
High-throughput screening assay; In vitro ssSTK autophosphorylation assay | |||
| Experiment for Drug Resistance |
Virulence assay; IC50 assay; Bacterial growth assay | |||
| Mechanism Description | In this study, we firstly identified the Thr167 and Ser175 residues in the activation loop of S. suis STK (ssSTK) as the kinase autophosphorylation sites. Phenotyping results demonstrated that the autophosphorylation deficient strain resembled the stk deletion strain showing essentiality for bacterial growth in minimal medium, abnormal morphology, and decreased virulence when compared with the wild-type S. suis SC19 strain. Based on these findings, we established an ssSTK inhibitor screening approach by measuring the growth of S. suis in a minimal medium and testing the autophosphorylation inhibition by measuring the consumption of ATP in an enzymatic reaction by ssSTK. A series of inhibitors against ssSTK are identified from a commercial kinase inhibitors library, including Staurosporine, K252a, AT9283, and APY29. These inhibitors showed antimicrobial activity in vitro. Moreover, by using Galleria mellonella larvae infection assay, compound APY29 displayed in vivo efficacy against S. suis infection. Additionally, it was predicted by molecular docking that these inhibitors could interact with ssSTK. Collectively, our data illustrated the essential roles of ssSTK autophosphorylation in the physiology and pathogenicity of S. suis and consider these inhibitors as promising antimicrobial lead compounds. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
