Drug Information
Drug (ID: DG01462) and It's Reported Resistant Information
| Name |
PD173074
|
||||
|---|---|---|---|---|---|
| Synonyms |
219580-11-7; PD173074; PD 173074; PD-173074; 1-(tert-Butyl)-3-(2-((4-(diethylamino)butyl)amino)-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl)urea; 1-tert-butyl-3-[2-{[4-(diethylamino)butyl]amino}-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]urea; UNII-A4TLL8634Y; A4TLL8634Y; CHEMBL189584; PD-0173074; CHEBI:63448; C28H41N7O3; MFCD08705327; 1-tert-butyl-3-(2-(4-(diethylamino)butylamino)-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl)urea; 1-tert-butyl-3-[2-[4-(diethylamino)butylamino]-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]urea; 1-tert-butyl-3-[6-(3,5-dimethoxy-phenyl)-2-(4-diethylamino-butylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea; 3-tert-butyl-1-(2-{[4-(diethylamino)butyl]amino}-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl)urea; 2fgi; SMR000568412; MLS001074892; MLS006011101; SCHEMBL177946; Pyrido[2,3-d]pyrimidine 12; BDBM6190; GTPL5037; AOB2517; DTXSID30176363; EX-A197; SYN1176; BCPP000121; HMS2233G17; HMS3265E09; HMS3265E10; HMS3265F09; HMS3265F10; HMS3371E08; HMS3648A10; HMS3654L09; BCP02368; ZINC3870533; NSC766908; s1264; AKOS016008595; BCP9001065; CCG-264881; CS-0182; NSC-766908; QC-7737; SB19382; NCGC00165863-01; NCGC00165863-02; NCGC00165863-17; AC-24850; AS-16310; BP162784; HY-10321; N-[2-[[4-(Diethylamino)butyl]amino] -6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-y l]-N'-(1,1-dimethylethyl)urea; FT-0673540; P2474; SW218104-2; X7486; PD 173074, >=96% (HPLC), powder; A25450; SR-01000837541; J-014372; J-523314; SR-01000837541-2; Q27088276; FGF/VEGF Receptor Tyrosine Kinase Inhibitor, PD173074 - CAS 219580-11-7; 1-(tert-Butyl)-3-[7-[[4-(diethylamino)butyl]amino]-3-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-2-yl]urea; 1-tert-Butyl-3-(6-(3,5-dimethoxyphenyl)-2-(4-diethylaminobutylamino)pyrido(2,3-d)pyrimidin-7-yl)urea; N-(tert-Butyl)-N -[2-[[4-(diethylamino)butyl]amino]-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]urea; N-[2-[[4-(Dethylamno)butyl]amno]-6-(3,5-dmethoxyphenyl)pyrdo[2,3-d ]pyrmdn-7-yl]-n'-(1,1-dmethylethyl)urea; N-[2-[[4-(Diethylamino)butyl]amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-N'-(1,1-dimethylethyl)urea; N-[2-[[4-(Diethylamino)butyl]amino]-6-(3,5- dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-N'-(1,1- dimethylethyl)urea; N-[2-[[4-(diethylamino)butyl]amino]-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-N'-(1,1-dimethylethyl)-urea; N-[2-[[4-(Diethylamino)butyl]amino]-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-N'-(1,1-dimethylethyl)urea; PD 173074;n-[2-[[4-(diethylamino)butyl]amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-n'-(1,1-dimethylethyl)urea
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
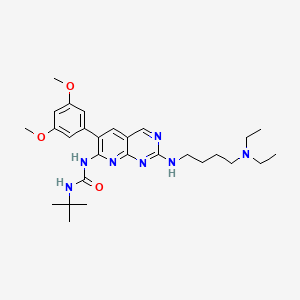
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[2]
|
||||
| Target | Long transient receptor potential channel 8 (TRPM8) | TRPM8_HUMAN | [2] | ||
| Transformation-sensitive protein p120 (TRPA1) | TRPA1_HUMAN | [2] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
13
|
||||
| IsoSMILES |
CCN(CC)CCCCNC1=NC2=NC(=C(C=C2C=N1)C3=CC(=CC(=C3)OC)OC)NC(=O)NC(C)(C)C
|
||||
| InChI |
InChI=1S/C28H41N7O3/c1-8-35(9-2)13-11-10-12-29-26-30-18-20-16-23(19-14-21(37-6)17-22(15-19)38-7)25(31-24(20)32-26)33-27(36)34-28(3,4)5/h14-18H,8-13H2,1-7H3,(H3,29,30,31,32,33,34,36)
|
||||
| InChIKey |
DXCUKNQANPLTEJ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.K660E (c.1978A>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
In vitro kinase inhibition assay | |||
| Mechanism Description | The missense mutation p.K660E (c.1978A>G) in gene FGFR2 cause the resistance of PD173074 by aberration of the drug's therapeutic target | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.M536I (c.1608G>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
In vitro kinase inhibition assay | |||
| Mechanism Description | The missense mutation p.M536I (c.1608G>C) in gene FGFR2 cause the resistance of PD173074 by aberration of the drug's therapeutic target | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.M538I (c.1614G>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
In vitro kinase inhibition assay | |||
| Mechanism Description | The missense mutation p.M538I (c.1614G>C) in gene FGFR2 cause the resistance of PD173074 by aberration of the drug's therapeutic target | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.I548V (c.1642A>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
In vitro kinase inhibition assay | |||
| Mechanism Description | The missense mutation p.I548V (c.1642A>G) in gene FGFR2 cause the resistance of PD173074 by aberration of the drug's therapeutic target | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.N550H (c.1648A>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
In vitro kinase inhibition assay | |||
| Mechanism Description | The missense mutation p.N550H (c.1648A>C) in gene FGFR2 cause the resistance of PD173074 by aberration of the drug's therapeutic target | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.N550S (c.1649A>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
In vitro kinase inhibition assay | |||
| Mechanism Description | The missense mutation p.N550S (c.1649A>G) in gene FGFR2 cause the resistance of PD173074 by aberration of the drug's therapeutic target | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.N550K (c.1650T>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
In vitro kinase inhibition assay | |||
| Mechanism Description | The missense mutation p.N550K (c.1650T>G) in gene FGFR2 cause the resistance of PD173074 by aberration of the drug's therapeutic target | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.V565I (c.1693G>A) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
In vitro kinase inhibition assay | |||
| Mechanism Description | The missense mutation p.V565I (c.1693G>A) in gene FGFR2 cause the resistance of PD173074 by aberration of the drug's therapeutic target | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.E566G (c.1697A>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
In vitro kinase inhibition assay | |||
| Mechanism Description | The missense mutation p.E566G (c.1697A>G) in gene FGFR2 cause the resistance of PD173074 by aberration of the drug's therapeutic target | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.L618M (c.1852T>A) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
In vitro kinase inhibition assay | |||
| Mechanism Description | The missense mutation p.L618M (c.1852T>A) in gene FGFR2 cause the resistance of PD173074 by aberration of the drug's therapeutic target | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Fibroblast growth factor receptor 4 (FGFR4) | [1] | |||
| Sensitive Disease | Alveolar rhabdomyosarcoma [ICD-11: 2B55.0] | |||
| Molecule Alteration | Missense mutation | p.N535K (c.1605C>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [3] | |||
| Sensitive Disease | Endometrial adenocarcinoma [ICD-11: 2C76.0] | |||
| Molecule Alteration | Missense mutation | p.N550K (c.1650T>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Uterus | N.A. | ||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Experiment for Drug Resistance |
Sulforhodamine B assay | |||
| Mechanism Description | The missense mutation p.N550K (c.1650T>A) in gene FGFR2 cause the sensitivity of PD173074 by aberration of the drug's therapeutic target | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
