Drug Information
Drug (ID: DG01182) and It's Reported Resistant Information
| Name |
Panobinostat
|
||||
|---|---|---|---|---|---|
| Synonyms |
Panobinostat; 404950-80-7; LBH589; LBH-589; Farydak; Panobinostat (LBH589); Faridak; LBH 589; (E)-N-Hydroxy-3-(4-(((2-(2-methyl-1H-indol-3-yl)ethyl)amino)methyl)phenyl)acrylamide; NVP-LBH589; NVP-LBH-589; UNII-9647FM7Y3Z; LBH-589B; CHEBI:85990; 9647FM7Y3Z; 404950-80-7 (free base); (E)-N-HYDROXY-3-(4-{[2-(2-METHYL-1H-INDOL-3-YL)-ETHYLAMINO]-METHYL}-PHENYL)-ACRYLAMIDE; (E)-N-hydroxy-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]prop-2-enamide; panobinostat hydrate; Farydak (TN); (2E)-N-hydroxy-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]acrylamide; (2E)-N-hydroxy-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enamide; (E)-N-hydroxy-3-(4-((2-(2-methyl-1H-indol-3-yl)ethylamino)methyl)phenyl)acrylamide; 2-Propenamide, N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-, (2E)-; Panobinostat [USAN:INN]; 2-Propenamide, N-hydroxy-3-(4-(((2-(2-methyl-1H-indol-3-yl)ethyl)amino)methyl)phenyl)-, (2E)-; Panobinostat(LBH589); LBH589 - Panobinostat; Panobinostat (USAN/INN); N-Hydroxy-3-[4-[2-(2-methyl-1H-indol-3-yl)ethylaminomethyl]phenyl]-2(E)-propenamide; MLS006011216; NVP-LBH 589; SCHEMBL164801; SCHEMBL183197; CHEMBL483254; GTPL7489; SCHEMBL22773814; BDBM29589; CHEBI:93774; DTXSID40193506; EX-A169; N-hydroxy-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]-2-propenamide; BCPP000187; BDBM198124; AOB87727; BCP01816; LBH58,9NVP-LBH589,Panobinostat; (LBH-589); MFCD09833242; NSC761190; s1030; ZINC22010649; AKOS005146046; BCP9000844; CCG-208762; CS-0267; DB06603; EX-8456; NSC-761190; (2E)-N-Hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide; (E)-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]prop-2-enehydroxamic acid; NCGC00263117-05; NCGC00263117-07; 2-Propenamide, N-hydroxy-3-(4-(((2-(2-methyl-1H-indol-3-yl)ethyl)amino) methyl)phenyl)-, (2E)-; AC-28652; AM808102; AS-17046; HY-10224; QC-10419; SMR004702978; DB-025426; SW219369-1; W6096; EC-000.2287; A25218; D10319; J-523585; Q7131441; BRD-K02130563-001-07-2; (E)-N-Hydroxy-3-[4-[[[2-(2-methyl-1H-indole-3-yl)ethyl]amino]methyl]phenyl]acrylamide; N-hydroxy-3 -[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide; N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2E-2-propenamide; Panobinostat;(E)-N-hydroxy-3-(4-((2-(2-methyl-1H-indol-3-yl)ethylamino)methyl)phenyl)acrylamide
Click to Show/Hide
|
||||
| Indication |
In total 3 Indication(s)
|
||||
| Structure |
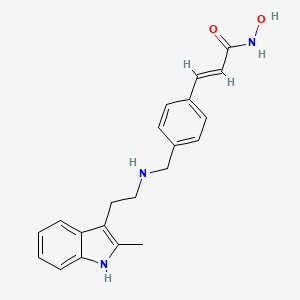
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | Histone deacetylase 1 (HDAC1) | HDAC1_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C21H23N3O2
|
||||
| IsoSMILES |
CC1=C(C2=CC=CC=C2N1)CCNCC3=CC=C(C=C3)/C=C/C(=O)NO
|
||||
| InChI |
1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+
|
||||
| InChIKey |
FPOHNWQLNRZRFC-ZHACJKMWSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Quinolinate phosphoribosyltransferase (QPRT) | [1] | |||
| Resistant Disease | Glioma [ICD-11: 2A00.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Glioma | |||
| The Studied Tissue | Brainstem tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.83E-01 Fold-change: 1.51E-03 Z-score: 2.53E-02 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | ES-2 cells | Ovary | Homo sapiens (Human) | CVCL_3509 |
| MG-63 cells | Bone | Homo sapiens (Human) | CVCL_0426 | |
| MMQ cells | Pituitary gland | Rattus norvegicus (Rat) | CVCL_2117 | |
| MOLM-13 cells | Peripheral blood | Homo sapiens (Human) | CVCL_2119 | |
| MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 | |
| SH-1-V8 cells | Esophagus | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
Western blot analysis; RNA-sequencing analysis | |||
| Experiment for Drug Resistance |
Flow cytometry | |||
| Mechanism Description | RNA-sequencing identifies quinolinic acid phosphoribosyltransferase (QPRT) as a highly expressed gene in bortezomib-panobinostat resistant U87 cells. QPRT, an enzyme catalyzing the rate-determining conversion of quinolinic acid (QA) to nicotinic acid mononucleotide (NAMN) a precursor for de novo NAD+ biosynthesis from tryptophan. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
