Drug Information
Drug (ID: DG01090) and It's Reported Resistant Information
| Name |
Sitagliptin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Sitagliptin; 486460-32-6; Januvia; Xelevia; MK-0431; (R)-3-AMINO-1-(3-(TRIFLUOROMETHYL)-5,6-DIHYDRO-[1,2,4]TRIAZOLO[4,3-A]PYRAZIN-7(8H)-YL)-4-(2,4,5-TRIFLUOROPHENYL)BUTAN-1-ONE; UNII-QFP0P1DV7Z; Tesavel; LEZ763; QFP0P1DV7Z; (3R)-3-AMINO-1-[3-(TRIFLUOROMETHYL)-5H,6H,7H,8H-[1,2,4]TRIAZOLO[4,3-A]PYRAZIN-7-YL]-4-(2,4,5-TRIFLUOROPHENYL)BUTAN-1-ONE; (3R)-3-amino-1-[3-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one; (2R)-4-OXO-4-[3-(TRIFLUOROMETHYL)-5,6-DIHYDRO[1,2,4]TRIAZOLO[4,3-A]PYRAZIN-7(8H)-YL]-1-(2,4,5-TRIFLUOROPHENYL)BUTAN-2-AMINE; 790712-60-6; CHEBI:40237; Sitagliptan; MK0431; Sitagliptin (Prop.INN); sitagliptina; sitagliptine; sitagliptinum; SR-05000001748; HSDB 7516; LEZ 763; (2R)-4-OXO-4-[3-(TRIFLUOROMETHYL)-5,6-DIHYDRO[1,2,4]TRIAZOLO[4,3-A]PYRAZIN-7(8H)-YL]-1-(2,4,5-TRIFLUOROPHENYL)BUTAN-2-A MINE; Sitagliptin (13); (3R)-3-Amino-1-[3-(trifluoromethyl)-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one; Sitagliptin; MK0431; 1169707-31-6; EC 690-730-1; CHEMBL1422; SCHEMBL17783; BSPBio_002262; Triazolopiperazine Analogue 1; (3R)-3-amino-1-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-4-(2,4,5-trifluorophenyl)butan-1-one; MLS006011959; Sitagliptin [USAN:INN:BAN]; GTPL6286; BDBM11162; C16H15F6N5O; AMY6930; DTXSID70197572; LEZ-763; 1x70; HMS2093F20; ACT02665; EX-A2816; Sitagliptin (Metformin,MK-0431); WHO 8692; ZINC1489478; MFCD09838015; NSC813215; AKOS015888724; CCG-268731; DB01261; MCULE-2101761133; NSC-813215; NCGC00178734-03; NCGC00178734-06; NCGC00178734-13; 7-((3R)-3-Amino-1-oxo-4-(2,4,5-trifluorophenyl)buyl)-5,6,7,8-tetrahydro-3-trifluoromethyl)-1,4-triazolo(4,3-a); AS-19118; HY-13749; SMR002546724; SBI-0206871.P001; Sitagliptin 100 microg/mL in Acetonitrile; S5079; X4663; A14377; A25516; D08516; AB01563393_01; AR-270/43507782; Q419832; Q-101366; Q-201711; SR-05000001748-1; BRD-K19416115-001-01-2; BRD-K19416115-001-03-8; Z1541638523; (1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorobenzyl)propylamine; (2r)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a] pyrazin-7(8h)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine; (2r)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8h)-yl]-1-(2,4,5-trifluorophenyl)butan-2-a; (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-alpha]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine; (3r)-3-amino-1-[3-(trifluoromethyl)-5,6,7,8-tetrahydro-5h-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one; (3R)-3-amino-1-[3-(trifluoromethyl)-5H,6H,7H,8H-[1,2,4]triazolo[3,4-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one hydrochloride; (R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine; 1,2,4-Triazolo(4,3-a)pyrazine, 7-((3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl)-5,6,7,8-tetrahydor-3-(trifluoromethyl)-; 1,2,4-Triazolo[4,3-a]pyrazine,7-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-; 3-oxo-1-(2,4,5-trifluorobenzyl)-3-(3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)propylamine; 7-[(3r)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazine
Click to Show/Hide
|
||||
| Indication |
In total 4 Indication(s)
|
||||
| Structure |
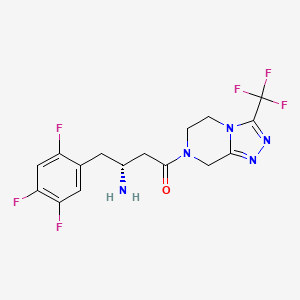
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C16H15F6N5O
|
||||
| IsoSMILES |
C1CN2C(=NN=C2C(F)(F)F)CN1C(=O)C[C@@H](CC3=CC(=C(C=C3F)F)F)N
|
||||
| InChI |
1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1
|
||||
| InChIKey |
MFFMDFFZMYYVKS-SECBINFHSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-05: Endocrine/nutritional/metabolic diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Dipeptidyl peptidase 4 (DPP4) | [1] | |||
| Sensitive Disease | Insulin-resistance syndrome [ICD-11: 5A44.0] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor that is currently indicated as an adjunctive treatment to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (DM). Sitagliptin is used to help mitigate insulin resistance after burn injury. | |||
ICD-08: Nervous system diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Protein kinase C gamma type (PRKCG) | [2] | |||
| Sensitive Disease | Epilepsy [ICD-11: 8A60.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vivo Model | Wistar rats model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
Protein kinase assay; RT-qPCR | |||
| Experiment for Drug Resistance |
Experimental Animal Model | |||
| Mechanism Description | Mechanistic insights revealed sitagliptin's ability to modulate the seizure grade and first myoclonic jerk latency via oxidative stress markers, like reduced glutathione and glutathione peroxidase emphasizing its antioxidative role in epilepsy. Additionally, it demonstrated anti-inflammatory effects by significantly reducing proinflammatory markers interleukin-1beta and interleukin-6. The modulation of key genes of the long-term potentiation pathway, particularly protein kinase C-gamma and metabotropic glutamate receptor 5, was evident through mRNA expression levels. Finally, sitagliptin showed potential neuroprotective properties, limiting pentylenetetrazolium-induced neuronal loss in the hippocampal region. Collectively, our findings suggest sitagliptin's multidimensional therapeutic potential for drug-resistant epilepsy specifically via a long-term potentiation pathway by inhibiting protein kinase C-gamma. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
