Drug Information
Drug (ID: DG01080) and It's Reported Resistant Information
| Name |
Maraviroc
|
||||
|---|---|---|---|---|---|
| Synonyms |
Maraviroc; 376348-65-1; Selzentry; Celsentri; UK-427857; UK-427,857; UK 427857; UNII-MD6P741W8A; MD6P741W8A; CHEMBL256907; 4,4-difluoro-N-[(1S)-3-[(1R,5S)-3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide; MVC; CHEMBL1201187; CHEBI:63608; NCGC00183109-02; 4,4-difluoro-N-((1S)-3-((1R,5S)-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexane-1-carboxamide; Celsentri(TM); Selzentry(TM); [3H]maraviroc; 4,4-difluoro-N-((1S)-3-((1R,5S)-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide; 4,4-Difluoro-N-[(1s)-3-{(3-Exo)-3-[3-Methyl-5-(Propan-2-Yl)-4h-1,2,4-Triazol-4-Yl]-8-Azabicyclo[3.2.1]oct-8-Yl}-1-Phenylpropyl]cyclohexanecarboxamide; PRO 140 & Maraviroc; maravirocum; Maraviroc [INN:BAN:JAN]; Rel-Maraviroc; C29H41F2N5O; HSDB 8021; 4,4-difluoro-N-[(1S)-3-[(1S,5R)-3-(3-isopropyl-5-methyl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenyl-propyl]cyclohexanecarboxamide; UK-427,857 maraviroc (MVC); [3H]UK 427,857; [3H]UK-427,857; (non-labelled)Maraviroc-d6; DSSTox_CID_28875; DSSTox_RID_83144; DSSTox_GSID_48949; SCHEMBL51991; GTPL803; GTPL806; Cyclohexanecarboxamide, 4,4-difluoro-N-((1S)-3-((3-exo)-3-(3-methyl-5-(1-methylethyl)-4H-1,2,4-triazol-4-yl)-8-azabicyclo(3.2.1)oct-8-yl)-1-phenylpropyl)-; Isopropyl, 4,4-difluoro-N-((1S)-3-{(1R,3s,5S)-3-(3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl)-8-azabicyclo(3.2.1)octan-8-yl}-1-phenylpropyl)cyclohexanecarboxamide; MLS006011960; CHEMBL584744; SCHEMBL2177194; SCHEMBL4576508; Maraviroc, >=98% (HPLC); EX-A200; CHEBI:184662; AMY12578; ZINC3817234; Tox21_113369; AC-558; BDBM50334986; BDBM50464147; MFCD13188530; AKOS025402143; AKOS032960315; ZINC100003902; ZINC101160855; CS-0366; DB04835; MCULE-2702173987; NCGC00183109-01; 4,4-Difluoro-N-[(1S)-3-[(3-exo)-3-[3-methyl-5-(1-methylethyl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl]cyclohexanecarboxamide; AS-75265; exo-4,4-Difluoro-N-[3-[3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1(S)-phenylpropyl]cyclohexanecarboxamide; HY-13004; PRO 140 (Anti-CCR5 monoclonal antibody) & exo-4,4-Difluoro-N-[3-[3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1(S)-phenylpropyl]cyclohexanecarboxamide; SMR004703532; CAS-376348-65-1; X5004; 348M651; Q421369; SR-01000942244; J-521678; SR-01000942244-1; BRD-A23284911-001-02-4; Z1618161028; 4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1S,5S)-3-(3-isopropyl-5-methyl-[1,2,4]triazol-4-yl)-8-aza-bicyclo[3.2.1]oct-8-yl]-1-phenyl-propyl}-amide; 4,4-difluoro-N-((1S)-3-(3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide; 4,4-Difluoro-N-((S)-3-((1R,3R,5S)-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexane-1-carboxamide; 4,4-difluoro-N-((S)-3-((1S,3R,5R)-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-aza-bicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide; 4,4-difluoro-N-((S)-3-((1S,3S,5R)-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-aza-bicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide; 4,4-difluoro-N-((S)-3-(3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-aza-bicyclo[3.2.1]octan-8-yl)-1-phenylpropyl)cyclohexanecarboxamide; 4,4-difluoro-N-[(1S)-3-[(1R,3S,5S)-3-[3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide; 4,4-difluoro-N-[(1S)-3-[(1R,5S)-3-(3-isopropyl-5-methyl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenyl-propyl]cyclohexanecarboxamide; 4,4-difluoro-N-[(1S)-3-[(1R,5S)-3-[3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide; 4,4-Difluoro-N-[(1S)-3-[(1R,5S)-3-[3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboximidic acid; 4,4-difluoro-N-{(1S)-3-[(3-exo)-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl}cyclohexanecarboxamide; 4,4-diluoro-N-[(1S)-3-[(1S,5R)-3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide; Cyclohexanecarboxamide,4,4-difluoro-N-[(1S)-3-[(3-exo)-3-[3-methyl-5-(1-methylethyl)-4H-1,2,4-triazol-4-yl]-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl]-; MRV
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
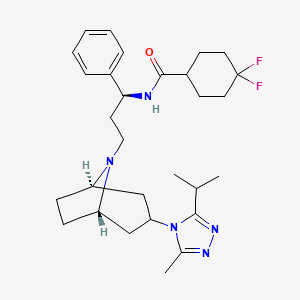
|
||||
| Target | C-C chemokine receptor type 5 (CCR5) | CCR5_HUMAN | [1] | ||
| CCR5 messenger RNA (CCR5 mRNA) | CCR5_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C29H41F2N5O
|
||||
| IsoSMILES |
CC1=NN=C(N1C2C[C@H]3CC[C@@H](C2)N3CC[C@@H](C4=CC=CC=C4)NC(=O)C5CCC(CC5)(F)F)C(C)C
|
||||
| InChI |
1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25 ,26-/m0/s1
|
||||
| InChIKey |
GSNHKUDZZFZSJB-HLMSNRGBSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: C-C motif chemokine receptor 5 (CCR5) | [1] | |||
| Sensitive Disease | Glioblastoma [ICD-11: 2A00.02] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| Cell Pathway Regulation | CCL5-CCR5 signaling pathway | Inhibition | has05163 | |
| In Vivo Model | Intracranial GBM patient-derived xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Neutral comet assay; ELISA assay; Immunofluorescence staining analysis; Immunohistochemistry staining analysi; Immunoblot assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Mechanism Description | The authors uncovered that pericytes potentiate DNA damage repair (DDR) in GBM cells residing in the perivascular niche, which induces temozolomide (TMZ) chemoresistance. Disrupting CCL5-CCR5 paracrine signaling through the brain-penetrable CCR5 antagonist maraviroc (MVC) potently inhibits pericyte-promoted DDR and effectively improves the chemotherapeutic efficacy of TMZ. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
