Drug Information
Drug (ID: DG01015) and It's Reported Resistant Information
| Name |
Deflazacort
|
||||
|---|---|---|---|---|---|
| Synonyms |
DEFLAZACORT; 14484-47-0; Azacort; Calcort; Oxazacort; Flantadin; Emflaza; Cortax; Deflan; MDL 458; UNII-KR5YZ6AE4B; C25H31NO6; DL-458-IT; L-5458; KR5YZ6AE4B; MDL-458; MFCD00866106; Dezacor; Lantadin; DSSTox_CID_378; DSSTox_RID_75552; DSSTox_GSID_20378; 2-((6aR,6bS,7S,8aS,8bS,11aR,12aS,12bS)-7-hydroxy-6a,8a,10-trimethyl-4-oxo-1,2,4,6a,6b,7,8,8a,11a,12,12a,12b-dodecahydro-8bH-naphtho[2',1':4,5]indeno[1,2-d]oxazol-8b-yl)-2-oxoethyl acetate; Deflazacortum; Decortil; Deflanil; Enzocort; Deflazacort (Calcort); 2-[(4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)-5-hydroxy-4a,6a,8-trimethyl-2-oxo-2,4a,4b,5,6,6a,9a,10,10a,10b,11,12-dodecahydro-6bH-naphtho[2',1':4,5]indeno[1,2-d][1,3]oxazol-6b-yl]-2-oxoethyl acetate; CAS-14484-47-0; Deflazacortum [INN-Latin]; Deflazacort [USAN:INN:BAN]; (11beta,16beta)-21-(Acetyloxy)-11-hydroxy-2'-methyl-5'H-pregna-1,4-dieno(17,16-d)oxazole-3,20-dione; (11beta,16beta)-21-(acetyloxy)-11-hydroxy-2'-methyl-5'H-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione; EINECS 238-483-7; Emflaza (TN); Deflazacort (USAN/INN); SCHEMBL4018; DL-458IT; GTPL9477; MDL458; CHEMBL1201891; DTXSID9020378; Deflazacort, >=98% (HPLC); CHEBI:135720; HMS3714D15; BCP08474; ZINC4212809; Tox21_112506; Tox21_301415; BBL036672; s1888; STL559051; AKOS015895199; Tox21_112506_1; CCG-220817; DB11921; KS-1158; NCGC00255189-01; NCGC00263521-01; 11beta,21-Dihydroxy-2'-methyl-5'betaH-pregna-1,4-dieno(17,16-d)oxazole-3,20-dione 21-acetate; H894; HY-13609; D4523; D03671; T70289; AB01274724-01; AB01274724_02; 484D470; Q779118; Q-101371; 3-AMINO-3-(4-CHLORO-3-NITRO-PHENYL)-PROPIONICACID; Deflazacort, United States Pharmacopeia (USP) Reference Standard; 11b,21-Dihydroxy-2'-methyl-5'bH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione 21-acetate; [2-[(1S,2S,4R,8S,9S,11S,12S,13R)-11-hydroxy-6,9,13-trimethyl-16-oxo-5-oxa-7-azapentacyclo[10.8.0.02,9.04,8.013,18]icosa-6,14,17-trien-8-yl]-2-oxoethyl] acetate; 11beta,21-dihydroxy-2'-methyl-5'betaH-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione 2'-acetate; 2-((6aR,6bS,7S,8aS,8bS,11aR,12aS,12bS)-7-hydroxy-6a,8a,10-trimethyl-4-oxo-2,4,6a,6b,7,8,8a,8b,11a,12,12a,12b-dodecahydro-1H-naphtho[2',1':4,5]indeno[1,2-d]oxazol-8b-yl)-2-oxoethyl acetate; 5'-beta-H-Pregna-1,4-dieno(17,16-d)oxazole-3,20-dione, 11-beta,21-dihydroxy-2'-methyl-, 21-acetate; 5'-beta-H-Pregna-1,4-dieno(17,16-d)oxazole-3,20-dione, 11-beta,21-dihydroxy-2'-methyl-,21-acetate; 5'H-Pregna-1,4-dieno(17,16-d)oxazole-3,20-dione, 21-(acetyloxy)-11-hydroxy-2'-methyl-, (11beta,16beta)-; pregna-1,4-diene-11beta,21-diol-3,20-dione[17alpha,16alpha-d]-2'-methyloxazoline 21-acetate
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
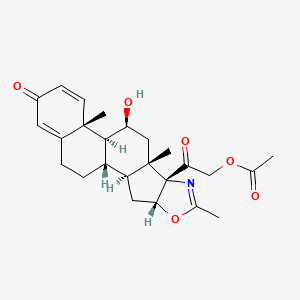
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
|
||||
| Target | Glucocorticoid receptor (NR3C1) | GCR_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C25H31NO6
|
||||
| IsoSMILES |
CC1=N[C@@]2([C@H](O1)C[C@@H]3[C@@]2(C[C@@H]([C@H]4[C@H]3CCC5=CC(=O)C=C[C@]45C)O)C)C(=O)COC(=O)C
|
||||
| InChI |
1S/C25H31NO6/c1-13-26-25(20(30)12-31-14(2)27)21(32-13)10-18-17-6-5-15-9-16(28)7-8-23(15,3)22(17)19(29)11-24(18,25)4/h7-9,17-19,21-22,29H,5-6,10-12H2,1-4H3/t17-,18-,19-,21+,22+,23-,24-,25+/m0/s1
|
||||
| InChIKey |
FBHSPRKOSMHSIF-GRMWVWQJSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-08: Nervous system diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: ADP/ATP translocase 2 (ADT2) | [1] | |||
| Resistant Disease | Duchenne muscular dystrophy [ICD-11: 8C70.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vivo Model | C57BL10 mouse model | Mus musculus | ||
| Mechanism Description | Deflazacort was found to reduce the resistance of skeletal mitochondria to MPT pore opening, which may be associated with a change in the level of ANT2 and CypD. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
