Drug Information
Drug (ID: DG00995) and It's Reported Resistant Information
| Name |
Valdecoxib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Valdecoxib; 181695-72-7; Bextra; 4-(5-methyl-3-phenylisoxazol-4-yl)benzenesulfonamide; Valdyn; SC 65872; SC-65872; 4-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonamide; Kudeq; 4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzenesulfonamide; Benzenesulfonamide, 4-(5-methyl-3-phenyl-4-isoxazolyl)-; YM-974; UNII-2919279Q3W; CHEMBL865; p-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonamide; 4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sulfonamide; CHEBI:63634; MFCD00950568; NSC-759846; COX; NCGC00095129-01; 2919279Q3W; DSSTox_CID_24226; DSSTox_RID_80128; DSSTox_GSID_44226; Valecoxib; SMR000466327; CAS-181695-72-7; HSDB 7302; Valdecoxib (USAN/INN); 4-[5-methyl-3-phenylisoxazol-4-yl]benzenesulfonamide; valdecoxibum; 4-(5-methyl-3-phenyl-isoxazol-4-yl)benzenesulfonamide; Valdecoxib [USAN:INN:BAN]; 4-(5-methyl-3-phenyl-4-isoxazolyl) benzenesulfonamide; ND-0214; Spectrum_001747; 2aw1; Spectrum2_000508; Spectrum3_001001; Spectrum4_001129; Spectrum5_001476; SCHEMBL3356; BSPBio_002721; KBioGR_001617; KBioSS_002227; MLS000759433; MLS001165699; MLS001195631; MLS001424105; SPECTRUM1504302; SPBio_000435; 4-(Methyl-3-phenyl-isoxazol-4-yl)-benzenesulfonamide; cid_119607; GTPL2894; ZINC6694; DTXSID6044226; Valdecoxib, >=98% (HPLC); BDBM13063; KBio2_002227; KBio2_004795; KBio2_007363; KBio3_001941; EX-A241; HMS1922J21; HMS2051P07; HMS2232P23; HMS3372D12; HMS3393P07; HMS3652I04; HMS3715L18; HMS3885O20; Pharmakon1600-01504302; AMY31078; BCP02419; Tox21_111438; AC-120; CCG-39589; NSC759846; s4049; AKOS000280920; Tox21_111438_1; CS-1674; DB00580; NC00184; NSC 759846; SB19519; MRF-0000216; NCGC00095129-02; NCGC00095129-03; NCGC00095129-06; Valdecoxib, analytical reference material; HY-15762; DB-044435; UNM-0000305814; FT-0631199; FT-0675755; SW197564-2; A13342; C21552; D02709; AB00639996-08; AB00639996_10; 695V727; A812629; Q347613; SR-01000759421; J-011613; J-513600; SR-01000759421-4; 4-[5-methyl-3-phenylisoxazol-4-yl]benzensulfonamide; BRD-K12994359-001-02-8; BRD-K12994359-001-14-3; 4-(5-Methyl-3-phenyl-isoxazol-4-yl)-benzenesulfonamide; Benzenesulfonamide, 4-(5-methyl-3-phenyl-4-isoxazolyl)- (9CI); VLX
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
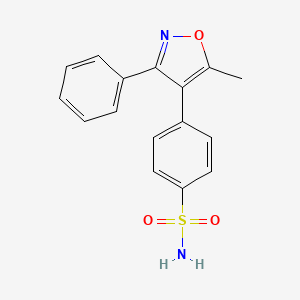
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(3 diseases)
[1]
[1]
[1]
|
||||
| Target | Prostaglandin G/H synthase 2 (COX-2) | PGH2_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C16H14N2O3S
|
||||
| IsoSMILES |
CC1=C(C(=NO1)C2=CC=CC=C2)C3=CC=C(C=C3)S(=O)(=O)N
|
||||
| InChI |
1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20)
|
||||
| InChIKey |
LNPDTQAFDNKSHK-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-15: Musculoskeletal/connective-tissue diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Prostaglandin G/H synthase 2 (Cox-2) | [1] | |||
| Resistant Disease | Rheumatoid arthritis [ICD-11: FA20.0] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| In Vivo Model | Standard diet fed male C57BL/6J (B6) mouse model; HFD fed male C57BL/6J (B6) mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Enzyme linked immunosorbent assay; Western blot analysis | |||
| Mechanism Description | Valdecoxib improves lipid-induced skeletal muscle insulin resistance via simultaneous suppression of inflammation and endoplasmic reticulum stress. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Prostaglandin G/H synthase 2 (Cox-2) | [1] | |||
| Resistant Disease | Osteoarthritis [ICD-11: FB84.2] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| In Vivo Model | Standard diet fed male C57BL/6J (B6) mouse model; HFD fed male C57BL/6J (B6) mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Enzyme linked immunosorbent assay; Western blot analysis | |||
| Mechanism Description | Valdecoxib improves lipid-induced skeletal muscle insulin resistance via simultaneous suppression of inflammation and endoplasmic reticulum stress. | |||
ICD-16: Genitourinary system diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Prostaglandin G/H synthase 2 (Cox-2) | [1] | |||
| Resistant Disease | Dysmenorrhea [ICD-11: GA34.3] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| In Vivo Model | Standard diet fed male C57BL/6J (B6) mouse model; HFD fed male C57BL/6J (B6) mouse model | Mus musculus | ||
| Experiment for Molecule Alteration |
Enzyme linked immunosorbent assay; Western blot analysis | |||
| Mechanism Description | Valdecoxib improves lipid-induced skeletal muscle insulin resistance via simultaneous suppression of inflammation and endoplasmic reticulum stress. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
