Drug Information
Drug (ID: DG00888) and It's Reported Resistant Information
| Name |
Zalcitabine
|
||||
|---|---|---|---|---|---|
| Synonyms |
Zalcitabine; Dideoxycytidine; 7481-89-2; 2',3'-DIDEOXYCYTIDINE; ddCyd; HIVID; Cytidine, 2',3'-dideoxy-; ddC; Zalcitibine; 4-Amino-1-((2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one; NSC 606170; Ro 24-2027/000; UNII-6L3XT8CB3I; CHEMBL853; 6L3XT8CB3I; 4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one; CHEBI:10101; MFCD00012188; NCGC00090705-08; DSSTox_CID_3747; NSC-606170; 4-AMINO-1-[(2R,5S)-5-(HYDROXYMETHYL)OXOLAN-2-YL]-1,2-DIHYDROPYRIMIDIN-2-ONE; DSSTox_RID_77182; DSSTox_GSID_23747; 2,3-dideoxycytidine; NSC606170; 4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2-one; SMR000058253; CCRIS 692; Hivid(TM); Hivid (TN); HSDB 7156; Interferon AD + ddC; ddC & GM-CSF; ddC & sCD4; PC-SOD & ddC; Ro-24-2027/000; DDC (DDC); BRN 0654956; Zalcitabine (JAN/USP/INN); DS-4152 & ddC; 1-(2,3-Dideoxy-beta-D-ribofuranosyl)cytosine; ddC & NP (from PHCA or HSA); SRI-7707; Zalcitabine [USAN:USP:INN:BAN]; CAS-7481-89-2; Zalcitabine- Bio-X; KS-1130; ddC & IFN.alpha.; .beta.-D-DDC; dideoxycytidine (DDC); 4-amino-1-[(2R,5S)-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one; Prestwick0_001037; Prestwick1_001037; Prestwick2_001037; Prestwick3_001037; ddC & Interferon.alpha.; 2', 3'-dideoxycytidine; bmse000712; UPCMLD-DP115; D 5782; SCHEMBL3598; TimTec1_004969; Lopac0_000360; BSPBio_001253; 3'-Azido-3'-deoxythymidine/2',3'-Dideoxycytidine; 5-25-14-00313 (Beilstein Handbook Reference); MLS000069636; MLS000759540; MLS001055363; MLS001424210; MLS006011951; SPBio_003104; BPBio1_001378; GTPL4828; ddC; ; ; 2',3'-Dideoxycytidine; DTXSID0023747; UPCMLD-DP115:001; ZINC39906; 2',3'-Dideoxycytidine & sCD4(soluble recombinant protein); AOB5609; HMS1548B19; HMS1571O15; HMS2051H18; HMS2090C12; HMS2098O15; HMS2236N08; HMS3261G21; HMS3715O15; Pharmakon1600-01502360; .beta.-D-2',3'-Dideoxycytidine; Zalcitabine, 2'3'-Dideoxycytidine; BCP13878; Tox21_113491; Tox21_201655; Tox21_303169; Tox21_500360; AC-824; BDBM50145605; Lecithinized superoxide dismutase & .beta.-D-2',3'-Dideoxycytidine; NSC759655; s1719; Sulfated polysaccharide-peptidoglycan DS-4152 & 2',3'-Dideoxycytidine; AKOS015854844; AKOS015894505; Tox21_113491_1; CCG-101050; CS-1110; DB00943; LP00360; MCULE-6071296177; NC00300; NSC-759655; SDCCGSBI-0050348.P002; 2',3'-Dideoxycytidine & Nanoparticles (from human serum albumin or polyhexylcyanoacrylate); SRI-7707-13; SRI-7707-14; SRI-7707_15; SRI-7707_17; NCGC00090705-01; NCGC00090705-02; NCGC00090705-03; NCGC00090705-05; NCGC00090705-06; NCGC00090705-07; NCGC00090705-09; NCGC00090705-10; NCGC00090705-11; NCGC00090705-13; NCGC00090705-15; NCGC00090705-24; NCGC00090705-25; NCGC00179242-01; NCGC00257202-01; NCGC00259204-01; NCGC00261045-01; BD164564; HY-17392; K278; ddC;Dideoxycytidine;2',3'-Dideoxycytidine; 2',3'-Dideoxycytidine & Interferon.alpha.; 2',3'-Dideoxycytidine, >=98% (HPLC); DB-019728; Ro-242027000; D3581; EU-0100360; Ro-24-2027000; SW197364-4; 2',3'-Dideoxycytidine, >=99.0% (HPLC); C07207; C76390; D00412; Cytidine, 2',3'-dideoxy- & Interferon.alpha.; 481D892; A838234; SR-01000075822; SR-01000736919; J-700276; Q-201941; Q2344582; SR-01000075822-1; SR-01000736919-5; Cytidine, 2',3'-dideoxy- & Colony-stimulating factor; Ro-242027000/Ro-24-2027-000; Z1550648753; Zalcitabine, United States Pharmacopeia (USP) Reference Standard; 4-Amino-1-(5-hydroxymethyl-tetrahydro-furan-2-yl)-1H-pyrimidin-2-one; .beta.-D-2',3'-Dideoxycytidine & Granulocyte-macrophage colony-stimulating factor
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
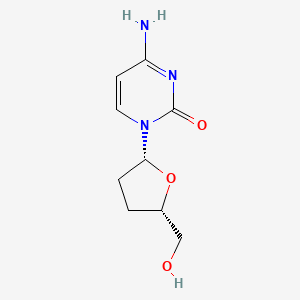
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Human immunodeficiency virus Reverse transcriptase (HIV RT) | POL_HV1B1 | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C9H13N3O3
|
||||
| IsoSMILES |
C1C[C@@H](O[C@@H]1CO)N2C=CC(=NC2=O)N
|
||||
| InChI |
1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1
|
||||
| InChIKey |
WREGKURFCTUGRC-POYBYMJQSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Gag-Pol polyprotein (POL) | [1] | |||
| Resistant Disease | Human immunodeficiency virus infection [ICD-11: 1C62.0] | |||
| Molecule Alteration | Mutation | . |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | Resistance to zalcitabine usually arises from a series of mutations within the HIV pol gene and develops less frequently than resistance to zidovudine. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
