Drug Information
Drug (ID: DG00867) and It's Reported Resistant Information
| Name |
Medroxyprogesterone
|
||||
|---|---|---|---|---|---|
| Synonyms |
Medroxyprogesterone; 520-85-4; Medroxyprogesteron; Medroxiprogesteronum; Medroxiprogesterona; Medroxyprogesteronum; 17-Hydroxy-6alpha-methylprogesterone; Hydroxymethylprogesterone; Medrossiprogesterone; 17alpha-Hydroxy-6alpha-methylprogesterone; 6alpha-Methyl-17alpha-hydroxyprogesterone; 6alpha-Methyl-4-pregnen-17alpha-ol-3,20-dione; 17-Hydroxy-6alpha-methyl-pregn-4-ene-3,20-dione; UNII-HSU1C9YRES; Medroxy Progesterone; U 8840; (6alpha)-17-hydroxy-6-methylpregn-4-ene-3,20-dione; HSU1C9YRES; (6S,8R,9S,10R,13S,14S,17R)-17-acetyl-17-hydroxy-6,10,13-trimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one; Pregn-4-ene-3,20-dione, 17-hydroxy-6-alpha-methyl-; MLS000069571; CHEBI:6715; 17alpha-Hydroxy-6alpha-methyl-4-pregnene-3,20-dione; SMR000058769; Medrossiprogesterone [DCIT]; NSC 27408; DSSTox_CID_16508; DSSTox_RID_79284; DSSTox_GSID_36508; Medroxyprogesteronum [INN-Latin]; Medroxiprogesterona [INN-Spanish]; Medroxyprogesterone [INN:BAN]; 17-Hydroxy-6.alpha.-methylprogesterone; 6-Dihydromegestrol; CAS-520-85-4; 17.alpha.-Hydroxy-6.alpha.-methylprogesterone; 6.alpha.-Methyl-17.alpha.-hydroxyprogesterone; HSDB 3114; 6-alpha-Methyl-17-alpha-hydroxyprogesterone; MLS002639162; EINECS 208-298-6; Farlutal inyectable (TN); Medroxyprogesterone (INN); BRN 2510965; NCGC00183122-01; CPD000058769; Pregn-4-ene-3,20-dione, 17-hydroxy-6-methyl-, (6alpha)-; Opera_ID_433; CHEMBL1390; 6alpha-Methyl-5-pregnen-17alpha-ol-3,20-dione; SCHEMBL37494; 4-08-00-02211 (Beilstein Handbook Reference); MLS001076098; MLS001424229; GTPL2879; DTXSID0036508; 17-Hydroxy-6a-methylprogesterone; HMS2052A13; HMS2230B08; HY-B0648; NSC27408; ZINC5763835; Tox21_113401; 3808AH; LMST02030176; MFCD00069474; s3635; AKOS015961681; Tox21_113401_1; CCG-101111; NC00361; MRF-0000022; NCGC00023064-04; NCGC00023064-05; AC-14528; M3113; 17; A-Hydroxy-6; A-methylprogesterone;U8840; Medroxyprogesterone acetate Related Compound B; C07119; C75441; D08166; Pregn-4-ene-3, 17-hydroxy-6.alpha.-methyl-; 520M854; A899740; Q416667; (6 )-17-Hydroxy-6-methylpregn-4-ene-3,20-dione; Medroxyprogesterone, VETRANAL(TM), analytical standard; Pregn-4-ene-3, 17-hydroxy-6-methyl-, (6.alpha.)-; Pregn-4-ene-3,20-dione, 17-hydroxy-6-methyl-, (6alpha)- (9CI); Pregn-4-ene-3,20-dione, 17-hydroxy-6-methyl-, (6-alpha)- (9CI); (6S,8R,10R,13S,17R)-17-Acetyl-17-hydroxy-6,10,13-trimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one; (6S,8R,9S,10R,13S,14S,17R)-17-acetyl-17-hydroxy-6,10,13-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3(2H)-one; Medroxyprogesterone acetate Related Compound B, United States Pharmacopeia (USP) Reference Standard
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
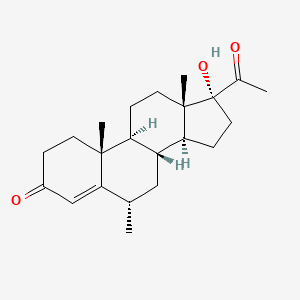
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | Progesterone receptor (PGR) | PRGR_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C22H32O3
|
||||
| IsoSMILES |
C[C@H]1C[C@@H]2[C@H](CC[C@]3([C@H]2CC[C@@]3(C(=O)C)O)C)[C@@]4(C1=CC(=O)CC4)C
|
||||
| InChI |
1S/C22H32O3/c1-13-11-16-17(20(3)8-5-15(24)12-19(13)20)6-9-21(4)18(16)7-10-22(21,25)14(2)23/h12-13,16-18,25H,5-11H2,1-4H3/t13-,16+,17-,18-,20+,21-,22-/m0/s1
|
||||
| InChIKey |
FRQMUZJSZHZSGN-HBNHAYAOSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Progesterone receptor (PGR) | [1] | |||
| Resistant Disease | Endometrial cancer [ICD-11: 2C76.1] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SR786 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1711 |
| IshikawaPR cells | Endometrium | Homo sapiens (Human) | CVCL_2529 | |
| Experiment for Molecule Alteration |
RTPCR | |||
| Mechanism Description | The presence of the progesterone receptor (PR) is the precondition for progesterone response and PR is a predictive marker for response of progesterone. Progesterone binds to its receptor PR-A and PR-B, subsequently inhibiting tumor growth and promoting tumor apoptosis by regulating downstream genes. Constant stimulation of progesterone reduced the expression of PGR and promoted the development of drug resistance. Thus, downregulation of PR especially PRB must be involved in progesterone resistance. However, the molecular mechanism of PGR dysfunction remains unclear. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
