Drug Information
Drug (ID: DG00847) and It's Reported Resistant Information
| Name |
Cycloserine
|
||||
|---|---|---|---|---|---|
| Synonyms |
D-cycloserine; cycloserine; 68-41-7; Seromycin; orientomycin; Oxamycin; Cyclo-D-serine; Cyclorin; D-4-amino-3-isoxazolidinone; Cicloserina; Farmiserina; Miroseryn; Tisomycin; Wasserina; Closina; Cycloserinum; D-4-amino-3-isoxazolidone; alpha-Cycloserine; (4R)-4-amino-1,2-oxazolidin-3-one; (+)-4-Amino-3-isoxazolidinone; Miroserina; Tebemicina; Novoserin; (R)-4-AMINOISOXAZOLIDIN-3-ONE; (+)-Cycloserine; Oxamicina; D-(+)-Cycloserine; (4R)-4-aminoisoxazolidin-3-one; PA 94; Cycloserin; Micoserina; PA-94; (R)-4-AMINO-ISOXAZOLIDIN-3-ONE; D-Oxamycin; RO-1-9213; D-CS; E-733-A; 3-Isoxazolidinone, 4-amino-, (4R)-; D-4-Amino-3-isossazolidone; 3-Isoxazolidinone, 4-amino-, (R)-; HSDB 3218; D-Oxamicina; 3-Isoxazolidinone, 4-amino-, d-; K-300; UNII-95IK5KI84Z; I-1431; 3-Isoxazolidinone, 4-amino-, (+)-; NSC 154851; CHEBI:40009; AI3-50153; D-Cycloserine, synthetic; DCS; (R)-(+)-4-Amino-3-isoxazolidinone; CHEMBL771; SC-49088; 95IK5KI84Z; MFCD00005353; CAS-68-41-7; NCGC00016306-01; Oxamicina [Italian]; Cicloserina [Italian]; DSSTox_CID_2870; 3-Isoxazolidinone, 4-amino-, (+)- (8CI); DSSTox_RID_76766; DSSTox_GSID_22870; Cycloserinum [INN-Latin]; Cicloserina [INN-Spanish]; Cycloserine, D-; Closerin; .alpha.-Cycloserine; (R)-Cycloserine; Seromycin (TN); SMR000058313; D-4-Amino-3-isossazolidone [Italian]; R-(+)-Cycloserine; (R)-4-Amino-3-isoxazolidinone; (4R)-4-Amino-3-isoxazolidinone; CYCLOSERINE (D); SR-01000075432; DRG-0195; (R)-(+)-Cycloserine; EINECS 200-688-4; D-amino-3-isoxazolidinone; BRN 0080798; (R)-4-Amino-3-isoxazolidone; NSC-76029; cycloserine-(d); Serine, cyclo-; NSC-154851; 3-Isoxazolidinone, 4-amino-, D; R(+)-4-Amino-3-isoxazolidinone; Cycloserine [USP:INN:BAN:JAN]; 4AX; 3-Isoxazolidinone, 4-amino-, (R); Cycloserine, D(+); D-Cycloserine, powder; Spectrum_000860; 1pb9; Prestwick0_001089; Prestwick1_001089; Prestwick2_001089; Prestwick3_001089; Spectrum2_000084; Spectrum3_000371; Spectrum4_000305; Spectrum5_000797; Lopac-C-1159; Lopac-C-3909; Lopac-C-7005; 3-Isoxazolidinone, 4-amino-, (4R)- (9CI); C 3909; C-9390; C-9400; Lopac0_000252; SCHEMBL34322; BSPBio_001138; BSPBio_002121; KBioGR_000890; KBioSS_001340; 4-27-00-05549 (Beilstein Handbook Reference); MLS000758215; MLS001423962; MLS002548887; BIDD:GT0707; D-Cycloserine synth. BP 88; DivK1c_000098; SPECTRUM1500215; SPBio_000008; SPBio_003029; BPBio1_001252; FA6C7F8B-D080-4EA3-978F-1ECFB5A29D09; GTPL9489; Cycloserine (JP17/USP/INN); 4-Amino-3-isoxazolidinone, D-; DTXSID8022870; HMS500E20; KBio1_000098; KBio2_001340; KBio2_003908; KBio2_006476; KBio3_001341; NINDS_000098; HMS1571I20; HMS1920C06; HMS2051C15; HMS2091I14; HMS2098I20; HMS2232F03; HMS3259L19; HMS3260D06; HMS3715I14; NJ-21; Pharmakon1600-01500215; (R)-3-Isoxazolidinone, 4-amino-; 4-Amino-3-isoxazolidinone, (R)-; ACT04767; HY-B0030; Tox21_110361; Tox21_500252; BDBM50038178; BDBM50103516; CCG-39705; D-Cycloserine, >=96.0% (NT); LMPK14000007; NSC756712; s1998; ZINC34676245; 4-Isoxazolidinamine, 3-oxo-, (D)-; AKOS015994626; Tox21_110361_1; AC-4721; DB00260; HS-0079; LP00252; MCULE-4212827696; NC00050; NC00676; NSC-756712; SDCCGSBI-0050240.P005; IDI1_000098; SMP1_000167; NCGC00015213-01; NCGC00015213-02; NCGC00015213-03; NCGC00016306-02; NCGC00016306-03; NCGC00016306-04; NCGC00016306-05; NCGC00016306-07; NCGC00016306-08; NCGC00016306-17; NCGC00093713-01; NCGC00093713-02; NCGC00260937-01; CAS-339-72-0; K138; SBI-0050240.P004; AB00443920; EU-0100252; C08057; D00877; AB00443920_09; AB00443920_10; 005C353; A836140; Q418508; SR-01000759389; SR-01000075432-1; SR-01000075432-2; SR-01000075432-5; SR-01000075432-9; SR-01000759389-4; SR-01000075432-10; F2173-1228; Z1522567171; Cycloserine, United States Pharmacopeia (USP) Reference Standard; Cycloserine, Pharmaceutical Secondary Standard; Certified Reference Material; (4R)-4-azaniumyl-4,5-dihydroisoxazol-3-olate;(R)-4-AMINOISOXAZOLIDIN-3-ONE
Click to Show/Hide
|
||||
| Indication |
In total 3 Indication(s)
|
||||
| Structure |
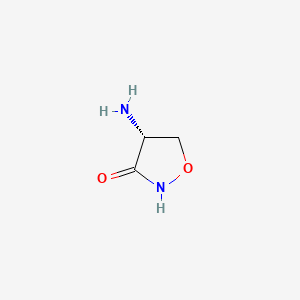
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[2]
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
|
||||
| Target | Glutamate receptor ionotropic NMDA 1 (NMDAR1) | NMDZ1_HUMAN | [1] | ||
| Mycobacterium Biosynthetic alanine racemase (MycB alr) | ALR_MYCSM | [1] | |||
| Mycobacterium D-alanine-D-alanine ligase A (MycB ddl) | DDL_MYCTU | [1] | |||
| N-methyl-D-aspartate receptor (NMDAR) | NOUNIPROTAC | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C3H6N2O2
|
||||
| IsoSMILES |
C1[C@H](C(=O)NO1)N
|
||||
| InChI |
1S/C3H6N2O2/c4-2-1-7-5-3(2)6/h2H,1,4H2,(H,5,6)/t2-/m1/s1
|
||||
| InChIKey |
DYDCUQKUCUHJBH-UWTATZPHSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Alanine racemase (ALR) | [1] | |||
| Resistant Disease | Tuberculosis [ICD-11: 1B10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | STK11 KO cells | Fetal kidney | Homo sapiens (Human) | CVCL_B3IE |
| Experiment for Drug Resistance |
Drug susceptibility testing | |||
| Mechanism Description | Since D-cycloserine is a structural analogue of D-alanine, enzymes with substrates of D-alanine are the drug targets of D-cycloserine in mycobacteria. These enzymes include D-alanine racemase (Alr) and D-alanine:D-alanine ligase (Ddl), which are required for the synthesis of peptidoglycan in the mycobacterial cell wall. Overexpression of alr and ddl has been shown to cause resistance to D-cycloserine in Mycobacterium smegmatis. Moreover, SNPs in these genes were also found in resistant Mycobacterium tuberculosis. Consistent with the cell-wall peptidoglycan being a target of D-cycloserine, previous studies have shown that D-cycloserine competitively inhibits both Alr and Ddl. However, a more recent metabolomic study showed that Ddl is a primary target of D-cycloserine and is preferentially inhibited over Alr in M. tuberculosis. | |||
| Key Molecule: D-alanine--D-alanine ligase (DDL) | [1] | |||
| Resistant Disease | Tuberculosis [ICD-11: 1B10.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | STK11 KO cells | Fetal kidney | Homo sapiens (Human) | CVCL_B3IE |
| Experiment for Drug Resistance |
Drug susceptibility testing | |||
| Mechanism Description | Since D-cycloserine is a structural analogue of D-alanine, enzymes with substrates of D-alanine are the drug targets of D-cycloserine in mycobacteria. These enzymes include D-alanine racemase (Alr) and D-alanine:D-alanine ligase (Ddl), which are required for the synthesis of peptidoglycan in the mycobacterial cell wall. Overexpression of alr and ddl has been shown to cause resistance to D-cycloserine in Mycobacterium smegmatis. Moreover, SNPs in these genes were also found in resistant Mycobacterium tuberculosis. Consistent with the cell-wall peptidoglycan being a target of D-cycloserine, previous studies have shown that D-cycloserine competitively inhibits both Alr and Ddl. However, a more recent metabolomic study showed that Ddl is a primary target of D-cycloserine and is preferentially inhibited over Alr in M. tuberculosis. | |||
| Key Molecule: Alanine racemase (ALR) | [1] | |||
| Resistant Disease | Tuberculosis [ICD-11: 1B10.0] | |||
| Molecule Alteration | Missense mutation | p.C1030T |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | STK11 KO cells | Fetal kidney | Homo sapiens (Human) | CVCL_B3IE |
| Experiment for Drug Resistance |
Drug susceptibility testing | |||
| Mechanism Description | Since D-cycloserine is a structural analogue of D-alanine, enzymes with substrates of D-alanine are the drug targets of D-cycloserine in mycobacteria. These enzymes include D-alanine racemase (Alr) and D-alanine:D-alanine ligase (Ddl), which are required for the synthesis of peptidoglycan in the mycobacterial cell wall. Overexpression of alr and ddl has been shown to cause resistance to D-cycloserine in Mycobacterium smegmatis. Moreover, SNPs in these genes were also found in resistant Mycobacterium tuberculosis. Consistent with the cell-wall peptidoglycan being a target of D-cycloserine, previous studies have shown that D-cycloserine competitively inhibits both Alr and Ddl. However, a more recent metabolomic study showed that Ddl is a primary target of D-cycloserine and is preferentially inhibited over Alr in M. tuberculosis. | |||
|
|
||||
| Key Molecule: ATP-binding cassette sub-family D member 1 (ABCD1) | [2] | |||
| Resistant Disease | Tuberculosis [ICD-11: 1B10.0] | |||
| Molecule Alteration | Mutation | rpsL gene at the 88th amino acid |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Experiment for Molecule Alteration |
GeneSeq assay; Bioinformatics assay | |||
| Mechanism Description | Out of total 112 mycobacterial positive cultures, five?M. bovis?were isolated and underwent WGS. All sequenced strains belonged to?Mycobacterium tuberculosis var bovis, spoligotype BOV_1; BOV_11. Resistance gene mutations were determined in 100% of strains to pyrazinamide (pncA?and?rpsA), isoniazid (KatG?and?ahpC), ethambutol (embB,?embC,?embR?and?ubiA), streptomycin (rpsl) and fluoroquinolones (gyrA?and?gyrB). Rifampin (rpoB?and?rpoC) and delamanid (fbiC) resistance genes were found in 80% of strains. The major represented virulence classes were the secretion system, cell surface components and regulation system. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
