Drug Information
Drug (ID: DG00777) and It's Reported Resistant Information
| Name |
Moclobemide
|
||||
|---|---|---|---|---|---|
| Synonyms |
Moclobemide; 71320-77-9; Aurorix; Moclobemid; Manerix; Moclamine; 4-chloro-N-(2-morpholinoethyl)benzamide; Moclaime; Moclobemidum; Moclamide; Moclobemida; Moclobemidum [INN-Latin]; Moclobemida [INN-Spanish]; p-Chloro-N-(2-morpholinoethyl)benzamide; 4-chloro-N-[2-(morpholin-4-yl)ethyl]benzamide; 4-Chlor-N-(2-morpholinoethyl)benzamid; 4-chloro-N-(2-morpholin-4-ylethyl)benzamide; 4-Chloro-N-(2-(4-morpholinyl)ethyl)benzamide; Ro 11-1163; 4-Chloro-N-(2-morpholin-4-yl-ethyl)-benzamide; Moclobemide (Ro 111163); GNF-PF-695; 4-chloro-N-[2-(4-morpholinyl)ethyl]benzamide; UNII-PJ0Y7AZB63; Ro 11-1163/000; Ro111163; BENZAMIDE, 4-CHLORO-N-(2-(4-MORPHOLINYL)ETHYL)-; Benzamide, 4-chloro-N-[2-(4-morpholinyl)ethyl]-; PJ0Y7AZB63; CHEMBL86304; MLS000070549; CHEBI:83531; MFCD00865388; NCGC00027930-02; NCGC00027930-04; SMR000012114; DSSTox_CID_20554; DSSTox_RID_79506; DSSTox_GSID_40554; Moclobemide [USAN:BAN:INN]; Moclobemide [USAN:INN:BAN]; Auromid; Aurorix (TN); CAS-71320-77-9; Ro-11-1163; HSDB 7180; SR-01000357772; CBMicro_048319; Moclobemide (USAN/INN); BRN 0530974; Moclobemide- Bio-X; Moclamine (Salt/Mix); Opera_ID_225; Oprea1_256739; Oprea1_270122; SCHEMBL49708; MLS000759438; MLS001240195; MLS001424077; GTPL7428; BENZAMIDE,4-CHLORO-N-[2-(4-MORPHOLINYL)ETHYL]-; DTXSID9040554; BDBM15613; AOB5012; HMS2051A16; HMS2096G07; HMS2232B20; HMS3262F09; HMS3371A01; HMS3393A16; HMS3657K05; HMS3713G07; HMS3885A15; AMY32534; BCP15783; HY-B0534; Moclobemide 1.0 mg/ml in Methanol; Tox21_110971; Tox21_113614; Tox21_500824; s3212; STK222240; ZINC19606670; AKOS003270184; Moclobemide, >=98% (HPLC), solid; Tox21_110971_1; CCG-100879; DB01171; LP00824; MCULE-5300106938; NC00129; SDCCGSBI-0048213.P003; NCGC00027930-03; NCGC00027930-05; NCGC00027930-07; NCGC00027930-16; NCGC00261509-01; AC-12467; BM164599; H223; p-chloro-N-(2-morpholinoethyl)-benzamide; BIM-0048213.P001; Ro-111163000; FT-0618228; M2733; SW197509-3; Ro-11-1163/000; D02561; AB00400932_12; 320M779; A837152; Q421934; 4-chloro-N-(2-morpholinoethyl)benzamide;Moclobemide; SR-01000357772-1; SR-01000357772-4; Z32409934; Ro11-1163; Ro 11-1163; Ro111163; Ro-111163; Ro 111163; 108375-13-9; MCL
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
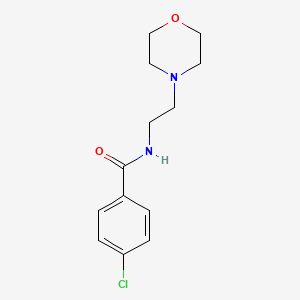
|
||||
| Target | Monoamine oxidase (MAO) | NOUNIPROTAC | [1] | ||
| Monoamine oxidase type A (MAO-A) | AOFA_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C13H17ClN2O2
|
||||
| IsoSMILES |
C1COCCN1CCNC(=O)C2=CC=C(C=C2)Cl
|
||||
| InChI |
1S/C13H17ClN2O2/c14-12-3-1-11(2-4-12)13(17)15-5-6-16-7-9-18-10-8-16/h1-4H,5-10H2,(H,15,17)
|
||||
| InChIKey |
YHXISWVBGDMDLQ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Monoamine oxidase A (MAOA) | [1] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | Monoamine oxidase A (MAOA) may promote tumor burden and drug/castration resistance in PCa. A positive association will pave the way for MAOA inhibitors such as moclobemide for PCa therapy. Association of key molecules of oncogenesis and metastasis with MAOA suggests that MAOA inhibitors such as moclobemide might be effective in the management of PCa. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
