Drug Information
Drug (ID: DG00752) and It's Reported Resistant Information
| Name |
Ifosfamide
|
||||
|---|---|---|---|---|---|
| Synonyms |
Ifosfamide; Isophosphamide; 3778-73-2; Iphosphamide; Isofosfamide; Ifosfamid; Mitoxana; Ifex; Iphosphamid; Isoendoxan; Naxamide; I-Phosphamide; Holoxan; Cyfos; Ifsofamide; Holoxan 1000; ASTA Z 4942; Ifosfamida; Ifosfamidum; MJF 9325; isosfamide; NCI-C01638; MJF-9325; NSC-109724; ifomide; Z4942; NSC 109724; A 4942; Z 4942; Z-4942; 2H-1,3,2-Oxazaphosphorin-2-amine, N,3-bis(2-chloroethyl)tetrahydro-, 2-oxide; C7H15Cl2N2O2P; NSC109724; 3-(2-Chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide; N,3-Bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylenephosphoric acid diamide; 3-(2-chloroethyl)-2-((2-chloroethyl)amino)-1,3,2-oxazaphosphinane 2-oxide; CHEBI:5864; Ifosphamide; 1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide; 3-(2-Chloroethyl)-2-(2-chloroethylamino)tetrahydro-2H-1,3,2-oxaazaphosphorin 2-oxide; 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2H-1,3,2-oxazaphosphorineoxide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylene phosphoric acid ester diamide; NCGC00016639-01; CAS-3778-73-2; DSSTox_CID_760; Ifosfamide Sterile; DSSTox_RID_75775; DSSTox_GSID_20760; Ifosfamidum [INN-Latin]; Ifosfamida [INN-Spanish]; (R)-3-(2-chloroethyl)-2-((2-chloroethyl)amino)-1,3,2-oxazaphosphinane 2-oxide; CCRIS 352; HSDB 7023; SR-05000002022; EINECS 223-237-3; IFEX (TN); BRN 0611835; Ifosfamide (JAN/USP/INN); N,3-bis(2-chloroethyl)-2-oxo-1,3,2 ^{5}-oxazaphosphinan-2-amine; Ifosfamid A; (R)-Ifosfamide; (S)-Ifosfamide; 3-(2-Chloroethyl)-2-((2-chloroethyl)amino)perhydro-2H-1,3,2-oxazaphosphorineoxide; 2,3-(N,N(sup 1)-Bis(2-chloroethyl)diamido)-1,3,2-oxazaphosphoridinoxyd; Isophosphamide,(S); N,3-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide; N,N-Bis(beta-chloroethyl)-amino-N',O-propylene-phosphoric acid ester diamide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylenephosphoric acid ester diamide; MFCD00057374; 3-(2-Chloroethyl)-2-((2-chloroethyl)amino)perhydro-2H-1,3,2-oxazaphosphorine oxide; N-(2-Chloraethyl)-N'-(2-chloraethyl)-N',O-propylen-phosphorsaureester-diamid [German]; Ifosfamide - Bio-X; Ifosfamide [USAN:USP:INN:BAN:JAN]; starbld0001221; Ifosfamide, >=98%; Prestwick0_000833; Prestwick1_000833; Prestwick2_000833; Prestwick3_000833; Intermediate of Ifosfamide; N-(2-Chloraethyl)-N'-(2-chloraethyl)-N',O-propylen-phosphorsaureester-diamid; SCHEMBL4885; 2H-1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide; CHEMBL1024; BSPBio_000785; MLS002154021; Ifex (TN) (Bristol Meyers); SPBio_002706; BPBio1_000865; GTPL7201; DTXSID7020760; (S)-3-(2-chloroethyl)-2-((2-chloroethyl)amino)-1,3,2-oxazaphosphinane 2-oxide; BDBM189358; HMS1570H07; HMS2090M12; HMS2093N07; HMS2097H07; HMS2232O10; HMS3374B08; HMS3654B15; HMS3714H07; Pharmakon1600-01505480; {3-(2-Chloroethyl)-2-[(2-; BCP06596; WLN: T6NPOTJ AM2G BO B2G; Tox21_110539; Tox21_201815; Tox21_302775; BBL028071; N,3-bis(2-chloroethyl)-2-oxo-1,3,2$l^{5}-oxazaphosphinan-2-amine; N,3-bis(2-chloroethyl)-2-oxo-1,3,2lambda5-oxazaphosphinan-2-amine; NSC759154; s1302; STL058690; AKOS005711213; Tox21_110539_1; AB02316; AC-2113; CCG-213464; CS-1424; DB01181; MCULE-1480299331; NSC-759154; N-(2-Chloroethyl)-N-(3-(2-chloroethyl)-2-oxido-1,3,2-oxazaphosphinan-2-yl)amine; Ifosfamide, analytical reference material; NCGC00179435-01; NCGC00179435-02; NCGC00179435-03; NCGC00179435-06; NCGC00179435-07; NCGC00256413-01; NCGC00259364-01; 2H-1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide (8CI); AS-10978; BI166243; HY-17419; NCI60_000233; SMR001233348; SBI-0206804.P001; DB-049196; AB00513932; FT-0603650; FT-0670282; SW197177-4; C07047; D00343; J10093; AB00513932-06; AB00513932-07; AB00513932-08; AB00513932_09; AB00513932_10; 778I732; A823873; Q418560; Q-101874; SR-05000002022-1; SR-05000002022-3; SR-05000002022-5; BRD-A67097164-001-11-2; Ifosfamide, British Pharmacopoeia (BP) Reference Standard; Ifosfamide, European Pharmacopoeia (EP) Reference Standard; UNII-UM20QQM95Y component HOMGKSMUEGBAAB-AWEZNQCLSA-N; Ifosfamide, United States Pharmacopeia (USP) Reference Standard; 2,N(sup 1)-Bis(2-chloroethyl)diamido-1,3,2-oxazaphosphoridinoxy-; 2,N(sup 1)-Bis(2-chloroethyl)diamido-1,3,2-oxazaphosphoridinoxyd; N,3-bis(2-chloroethyl)-2-oxo-1,3,2$l;{5}-oxazaphosphinan-2-amine; {3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2H-1,3,} 2-oxazaphosphorine oxide; 1,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-, 2-oxide; 2H-1,2-Oxazaphosphorin-2-amine, N,3-bis(2-chloroethyl)tetrahydro-, 2-oxide; 2H-1,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-, 2-oxide; 3-(2-Chloroethyl)-2-(2-chloroethylamino)tetrahydro-2H-1,3,2-oxazaphosphorin-2-one; 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]-1,3,2$l^{5}-oxazaphosphinan-2-one; 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]-1,3,2lambda5-oxazaphosphinan-2-one; 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2H-1,2-oxazaphosphorine oxide; 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2H-1,2-oxazaphosphorineoxide; 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-2H-1,2-oxazaphosphorine 2-oxide; N,3-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphinan-2-amine 2-oxide #
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
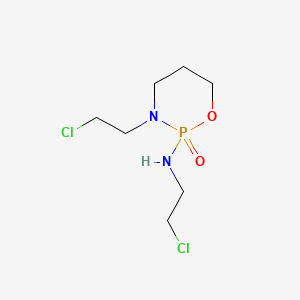
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Human Deoxyribonucleic acid (hDNA) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C7H15Cl2N2O2P
|
||||
| IsoSMILES |
C1CN(P(=O)(OC1)NCCCl)CCCl
|
||||
| InChI |
1S/C7H15Cl2N2O2P/c8-2-4-10-14(12)11(6-3-9)5-1-7-13-14/h1-7H2,(H,10,12)
|
||||
| InChIKey |
HOMGKSMUEGBAAB-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tripartite motif containing 37 (TRIM37) | [1] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Wnt/Beta-catenin signaling pathway | Activation | hsa04310 | |
| In Vitro Model | Saccharomyces cerevisiae strain BY4742 | 4932 | ||
| SH-1-V6 cells | Esophagus | Homo sapiens (Human) | N.A. | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Mechanism Description | High expression of TRIM37 mRNA and protein was observed in paediatric osteosarcoma tumour samples. Treatment of cell lines Saos-2 and MG-63 with ifosfamide, doxorubicin, cisplatin, or methotrexate induced TRIM37 expression. Upregulation of TRIM37 in vitro induced drug resistance, whereas TRIM37 knockdown restored chemosensitivity. The TRIM37-induced chemoresistance was found to be partially dependent on the activation of the Wnt/beta-catenin signalling pathway. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
