Drug Information
Drug (ID: DG00748) and It's Reported Resistant Information
| Name |
Ibudilast
|
||||
|---|---|---|---|---|---|
| Synonyms |
Ibudilast; 50847-11-5; Ketas; KC-404; Ibudilastum; Ke Tas; Ibudilastum [Latin]; MN-166; Ibudilast [INN:JAN]; Eyevinal; AV-411; UNII-M0TTH61XC5; Ketas (TN); 3-Isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine; 2-methyl-1-(2-propan-2-ylpyrazolo[1,5-a]pyridin-3-yl)propan-1-one; 1-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-2-methylpropan-1-one; 3-Isobutyryl-2-isopropylpyrazolo(1,5-a)pyridine; Tocris-1694; Lopac-I-0157; 1-Propanone, 2-methyl-1-[2-(1-methylethyl)pyrazolo[1,5-a]pyridin-3-yl]-; 2-Isopropyl-3-isobutyrylpyrazolo(1,5-a)pyridine; AV411; MFCD00864808; M0TTH61XC5; CHEMBL19449; KC-404;AV-411;MN-166; NCGC00015542-05; 2-Methyl-1-[2-(Propan-2-Yl)pyrazolo[1,5-A]pyridin-3-Yl]propan-1-One; 1-Propanone, 2-methyl-1-(2-(1-methylethyl)pyrazolo(1,5-a)pyridin-3-yl)-; DSSTox_CID_28933; DSSTox_RID_83199; 2-isopropyl-3-isobutyrylpyrazolo[1,5-a]pyridine; DSSTox_GSID_49007; Ibudilast (JAN/INN); CAS-50847-11-5; SR-01000075927; AV 411; BRN 0656579; Pinatos; I0157_SIGMA; Ibudilast,(S); Pyrazolo(1,5-a)pyridine, 3-isobutyryl-2-isopropyl-; 1-(2-Isopropylpyrazolo(1,5-a)pyridin-3-yl)-2-methyl-1-propanone; Ibudilast (JP17/INN); I 0157; Lopac0_000599; SCHEMBL30390; 5-24-03-00396 (Beilstein Handbook Reference); MLS000862198; GTPL7399; ZINC4234; DTXSID7049007; CHEBI:31684; BCPP000209; HMS2089B21; HMS2233H08; HMS3261H20; HMS3268O11; HMS3374P02; HMS3412B20; HMS3676B20; HMS3715L09; HMS3886M03; BCP02335; HY-B0763; Tox21_113503; Tox21_500599; BDBM50240404; s4837; Ibudilast, >=99% (HPLC), solid; 2-Methyl-1-[2-(1-methylethyl)pyrazolo[1,5-a]pyridin-3-yl] 1-propanone; AKOS015895123; Tox21_113503_1; AC-1044; BCP9000768; CCG-204688; DB05266; LP00599; SB19092; SDCCGSBI-0050581.P002; NCGC00015542-01; NCGC00015542-02; NCGC00015542-03; NCGC00015542-04; NCGC00015542-06; NCGC00015542-07; NCGC00015542-17; NCGC00025261-01; NCGC00025261-02; NCGC00025261-03; NCGC00025261-04; NCGC00261284-01; SMR000326961; SY051343; EU-0100599; FT-0654591; FT-0670255; I0740; D01385; F20666; AB00698306-06; 3-Isobutyryl-2-isopropyl-Pyrazolo(1,5-a)pyridine; 847I115; A828320; H-20256; L003042; Q261167; J-512714; SR-01000075927-1; SR-01000075927-3; SR-01000075927-6; BRD-K16444452-001-03-4; 1-(2-isopropylH-pyrazolo[1,5-a]pyridin-3-yl)-2-methylpropan-1-one; 1-(2-Isopropyl-pyrazolo[1,5-a]pyridin-3-yl)-2-methyl-propan-1-one; 1-(2-Isopropyl-pyrazolo[1,5-alpha]pyridin-3-yl)-2-methyl-propan-1-one; 1-(2-isopropylpyrazolo[1,5-alpha]pyridin-3-yl)-2-methylpropan-1-one; 2-methyl-1-(2-propan-2-yl-3-pyrazolo[1,5-a]pyridinyl)-1-propanone; 2-methyl-1-[2-(1-methylethyl)pyrazolo[1,5-a]pyridin-3-yl]-1-propanone; (Ibudilast)1-(2-Isopropyl-pyrazolo[1,5-a]pyridin-3-yl)-2-methyl-propan-1-one; 1-(2-Isopropylpyrazolo[1,5-a]pyridin-3-yl)-2-methylpropan-1-one (Ibudilast); AVL
Click to Show/Hide
|
||||
| Indication |
In total 6 Indication(s)
|
||||
| Structure |
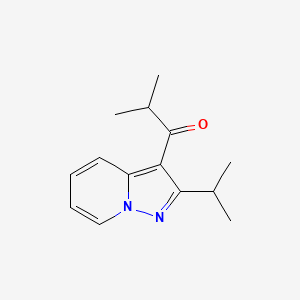
|
||||
| Target | Phosphodiesterase 3 (PDE3) | NOUNIPROTAC | [1] | ||
| Phosphodiesterase 4 (PDE4) | NOUNIPROTAC | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C14H18N2O
|
||||
| IsoSMILES |
CC(C)C1=NN2C=CC=CC2=C1C(=O)C(C)C
|
||||
| InChI |
1S/C14H18N2O/c1-9(2)13-12(14(17)10(3)4)11-7-5-6-8-16(11)15-13/h5-10H,1-4H3
|
||||
| InChIKey |
ZJVFLBOZORBYFE-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Toll like receptor 4 (TLR4) | [1] | |||
| Sensitive Disease | Bone infection [ICD-11: 1B2Z.9] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vivo Model | Femoral defect model; Male C57BL/6NCrlBltw mouse model | Mus musculus | ||
| Experiment for Drug Resistance |
Serum Osteocalcin and CTX1 Assay; Micro-CT Bone Imaging | |||
| Mechanism Description | LPS inhibited osteogenic factor-induced MC3T3-E1 cell differentiation, alkaline phosphatase (ALP) levels, calcium deposition, and osteopontin secretion and increased the activity of osteoclast-associated molecules, including cathepsin K and tartrate-resistant acid phosphatase in vitro. Ibudilast blocked the LPS-induced inhibition of osteoblast activation and activation of osteoclast in vitro and attenuated LPS-induced delayed callus bone formation in vivo. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
