Drug Information
Drug (ID: DG00582) and It's Reported Resistant Information
| Name |
Bimatoprost
|
||||
|---|---|---|---|---|---|
| Synonyms |
Bimatoprost; 155206-00-1; Lumigan; Latisse; AGN 192024; UNII-QXS94885MZ; AGN-192024; (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-enamide; QXS94885MZ; CHEBI:51230; (Z)-7-((1R,2R,3R,5S)-3,5-Dihydroxy-2-((1E,3S)-3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-N-ethyl-5-heptenamide; (E)-7-[3,5-dihydroxy-2-[(E)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-N-ethylhept-5-enamide; bimatoprostum; Prostamide; (5Z)-7-{(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenylpent-1-en-1-yl]cyclopentyl}-N-ethylhept-5-enamide; (5z)-7-{(1r,2r,3r,5s)-3,5-Dihydroxy-2-[(1e,3s)-3-Hydroxy-5-Phenylpent-1-Enyl]cyclopentyl}-N-Ethylhept-5-Enamide; Lumigan (TN); (5Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenylpent-1-en-1-yl]cyclopentyl]-N-ethylhept-5-enamide; Bimatoprost [USAN:INN:BAN:JAN]; Latisse (TN); LS-181817; SCHEMBL24425; 5-Heptenamide, 7-(3,5-dihydroxy-2-(3-hydrdoxy-5-phenyl-1-pentenyl)cyclopentyl)-N-ethyl-, (1R-(1alpha(Z),2beta(1E,3S*),3alpha,5alpha))-; MLS006010039; US9271961, Bimatoprost; Bimatoprost (JAN/USAN/INN); GTPL1958; CHEMBL1200963; DTXSID30895042; BDBM220120; EX-A1769; HY-B0191; ZINC4474405; MFCD03411999; AKOS015995566; AM84507; DB00905; NCGC00181745-01; NCGC00181745-03; 5-Heptenamide, 7-((1R,2R,3R,5S)-3,5-dihydroxy-2-((1E,3S)-3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-N-ethyl-, (5Z)-; 5-Heptenamide, 7-(3,5-dihydroxy-2-(3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-N-ethyl-, (1R-1(alpha(Z),2beta(1E,3S*)3alpha,5alpha))-; AS-35082; M052; SMR000058996; D02724; 206B001; SR-01000942224; Q2393348; SR-01000942224-1; 17-PHENYL TRINOR PROSTAGLANDIN F2ALPHA ETHYL AMIDE; 17-Phenyl-tri-norprostaglandin F2alpha-ethyl amide, >=95%, solid; 15M; 5-Heptenamide, 7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]cyclopentyl]-N-ethyl-, (5Z)-
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
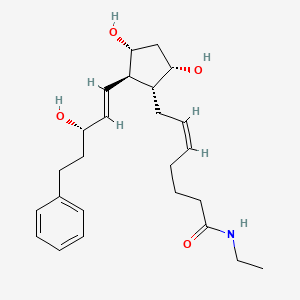
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Prostaglandin receptor (PTGR) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C25H37NO4
|
||||
| IsoSMILES |
CCNC(=O)CCC/C=C\\C[C@H]1[C@H](C[C@H]([C@@H]1/C=C/[C@H](CCC2=CC=CC=C2)O)O)O
|
||||
| InChI |
1S/C25H37NO4/c1-2-26-25(30)13-9-4-3-8-12-21-22(24(29)18-23(21)28)17-16-20(27)15-14-19-10-6-5-7-11-19/h3,5-8,10-11,16-17,20-24,27-29H,2,4,9,12-15,18H2,1H3,(H,26,30)/b8-3-,17-16+/t20-,21+,22+,23-,24+/m0/s1
|
||||
| InChIKey |
AQOKCDNYWBIDND-FTOWTWDKSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-09: Visual system diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: ATP-binding cassette sub-family C5 (ABCC5) | [1] | |||
| Resistant Disease | Glaucoma [ICD-11: 9C61.0] | |||
| Molecule Alteration | Expression | Regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | SIRC cells | Colon | Homo sapiens (Human) | CVCL_2724 |
| SV40-HCEC cells | Kidney | Homo sapiens (Human) | CVCL_1272 | |
| Experiment for Molecule Alteration |
RT-PCR; Immunoprecipitation assay; Western blot analysis; Immunostaining assay | |||
| Experiment for Drug Resistance |
Trypan blue exclusion test assay | |||
| Mechanism Description | Taken together immunolocalization on human cornea, in vitro efflux in human, rabbit corneal and MRP5 over expressing cells, ex vivo and in vivo studies in intact rabbit cornea suggest that MRP5 on cornea can significantly lower the permeability of antiviral and glaucoma drugs. | |||
| Key Molecule: ATP-binding cassette sub-family C5 (ABCC5) | [1] | |||
| Resistant Disease | Glaucoma [ICD-11: 9C61.0] | |||
| Molecule Alteration | Expression | Regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | SIRC cells | Colon | Homo sapiens (Human) | CVCL_2724 |
| SV40-HCEC cells | Kidney | Homo sapiens (Human) | CVCL_1272 | |
| Experiment for Molecule Alteration |
RT-PCR; Immunoprecipitation assay; Western blot analysis; Immunostaining assay | |||
| Experiment for Drug Resistance |
Trypan blue exclusion test assay | |||
| Mechanism Description | Taken together immunolocalization on human cornea, in vitro efflux in human, rabbit corneal and MRP5 over expressing cells, ex vivo and in vivo studies in intact rabbit cornea suggest that MRP5 on cornea can significantly lower the permeability of antiviral and glaucoma drugs. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
