Drug Information
Drug (ID: DG00581) and It's Reported Resistant Information
| Name |
TAS-120
|
||||
|---|---|---|---|---|---|
| Synonyms |
Futibatinib; TAS-120; 1448169-71-8; FGFR-IN-1; TAS120; UNII-4B93MGE4AL; 4B93MGE4AL; TAS 120; 1-[(3S)-3-[4-amino-3-[2-(3,5-dimethoxyphenyl)ethynyl]-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-1-pyrrolidinyl]-2-propen-1-one; 1-[(3S)-3-[4-amino-3-[2-(3,5-dimethoxyphenyl)ethynyl]pyrazolo[3,4-d]pyrimidin-1-yl]pyrrolidin-1-yl]prop-2-en-1-one; 1-[(3S)-3-{4-amino-3-[(3,5-dimethoxyphenyl)ethynyl]-1H-pyrazolo[3,4-d]pyrimidin-1-yl}pyrrolidin-1-yl]prop-2-en-1-one; Futibatinib [USAN]; Futibatinib (JAN/USAN/INN); GTPL9786; CHEMBL3701238; SCHEMBL15345470; TAS 120 [WHO-DD]; BDBM161389; AMY16930; BCP17213; EX-A1862; NSC813488; WHO 10879; ZINC207800318; CS-6031; DB15149; Example 2 [WO2013108809]; NSC-813488; (S)-1-(3-(4-amino-3-((3,5-dimethoxyphenyl)ethynyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)pyrrolidin-1-yl)prop-2-en-1-one; BS-15425; HY-100818; D11725; D77976; US9108973, 2; 2-Propen-1-one, 1-((3S)-3-(4-amino-3-(2-(3,5-dimethoxyphenyl)ethynyl)-1H-pyrazolo(3,4-d)pyrimidin-1-yl)-1-pyrrolidinyl)-
Click to Show/Hide
|
||||
| Indication |
In total 3 Indication(s)
|
||||
| Structure |
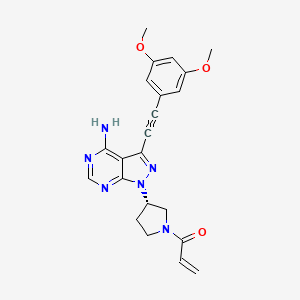
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Fibroblast growth factor (FGF) | NOUNIPROTAC | [1] | ||
| Fibroblast growth factor receptor (FGFR) | NOUNIPROTAC | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C22H22N6O3
|
||||
| IsoSMILES |
COC1=CC(=CC(=C1)C#CC2=NN(C3=NC=NC(=C23)N)[C@H]4CCN(C4)C(=O)C=C)OC
|
||||
| InChI |
1S/C22H22N6O3/c1-4-19(29)27-8-7-15(12-27)28-22-20(21(23)24-13-25-22)18(26-28)6-5-14-9-16(30-2)11-17(10-14)31-3/h4,9-11,13,15H,1,7-8,12H2,2-3H3,(H2,23,24,25)/t15-/m0/s1
|
||||
| InChIKey |
KEIPNCCJPRMIAX-HNNXBMFYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [1] | |||
| Resistant Disease | Intrahepatic cholangiocarcinoma [ICD-11: 2C12.1] | |||
| Molecule Alteration | Missense mutation | p.V565F |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
ctDNA analysis | |||
| Mechanism Description | TAS-120 overcomes resistance to atp-competitive fgfr inhibitors in patients with fgfr2 fusion-positive intrahepatic cholangiocarcinoma. | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [1] | |||
| Resistant Disease | Intrahepatic cholangiocarcinoma [ICD-11: 2C12.1] | |||
| Molecule Alteration | Missense mutation | p.V565F |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
ctDNA analysis | |||
| Mechanism Description | TAS-120 overcomes resistance to atp-competitive fgfr inhibitors in patients with fgfr2 fusion-positive intrahepatic cholangiocarcinoma. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [1] | |||
| Sensitive Disease | Intrahepatic cholangiocarcinoma [ICD-11: 2C12.1] | |||
| Molecule Alteration | Missense mutation | p.V565I |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
ctDNA analysis | |||
| Mechanism Description | TAS-120 overcomes resistance to atp-competitive fgfr inhibitors in patients with fgfr2 fusion-positive intrahepatic cholangiocarcinoma. | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [1] | |||
| Sensitive Disease | Intrahepatic cholangiocarcinoma [ICD-11: 2C12.1] | |||
| Molecule Alteration | Missense mutation | p.N550H |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
ctDNA analysis | |||
| Mechanism Description | TAS-120 overcomes resistance to atp-competitive fgfr inhibitors in patients with fgfr2 fusion-positive intrahepatic cholangiocarcinoma. | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [1] | |||
| Sensitive Disease | Intrahepatic cholangiocarcinoma [ICD-11: 2C12.1] | |||
| Molecule Alteration | Missense mutation | p.E566A |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
ctDNA analysis | |||
| Mechanism Description | TAS-120 overcomes resistance to atp-competitive fgfr inhibitors in patients with fgfr2 fusion-positive intrahepatic cholangiocarcinoma. | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [1] | |||
| Sensitive Disease | Intrahepatic cholangiocarcinoma [ICD-11: 2C12.1] | |||
| Molecule Alteration | Missense mutation | p.V565I |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
ctDNA analysis | |||
| Mechanism Description | TAS-120 overcomes resistance to atp-competitive fgfr inhibitors in patients with fgfr2 fusion-positive intrahepatic cholangiocarcinoma. | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [1] | |||
| Sensitive Disease | Intrahepatic cholangiocarcinoma [ICD-11: 2C12.1] | |||
| Molecule Alteration | Missense mutation | p.N550H |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
ctDNA analysis | |||
| Mechanism Description | TAS-120 overcomes resistance to atp-competitive fgfr inhibitors in patients with fgfr2 fusion-positive intrahepatic cholangiocarcinoma. | |||
| Key Molecule: Fibroblast growth factor receptor 2 (FGFR2) | [1] | |||
| Sensitive Disease | Intrahepatic cholangiocarcinoma [ICD-11: 2C12.1] | |||
| Molecule Alteration | Missense mutation | p.E566A |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
ctDNA analysis | |||
| Mechanism Description | TAS-120 overcomes resistance to atp-competitive fgfr inhibitors in patients with fgfr2 fusion-positive intrahepatic cholangiocarcinoma. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
