Drug Information
Drug (ID: DG00406) and It's Reported Resistant Information
| Name |
Sulfathiazole
|
||||
|---|---|---|---|---|---|
| Synonyms |
Sulfathiazole; 72-14-0; Sulphathiazole; Sulfathiazol; 2-Sulfanilamidothiazole; Sulfanilamidothiazole; Thiazamide; Norsulfasol; Norsulfazole; 2-Sulfonamidothiazole; 4-Amino-N-(thiazol-2-yl)benzenesulfonamide; 2-(Sulfanilylamino)thiazole; Neostrepsan; Sulfocerol; Thiozamide; Sulzol; 2-Sulfanilamidothiazol; 2-(p-Aminobenzenesulfonamido)thiazole; Azoquimiol; Azoseptale; Norsulfazol; Poliseptil; Sanotiazol; Sulfathiazolum; Sulfatiazol; Thiacoccine; Thiasulfol; Wintrazole; Cerazole; Chemosept; Cibazol; Eleudron; Estafilol; Planomide; Septozol; Duatok; Dulana; N(1)-2-Thiazolylsulfanilamide; Coco-Thiazole; Formosulfathiazole; Streptosilthiazole; Sulfamul; 2-(p-Aminobenzenesulphonamido)thiazole; Usaf sn-9; 4-Amino-N-2-thiazolylbenzenesulfonamide; Cerazol (suspension); Ciba 3714; 4-Amino-N-(1,3-thiazol-2-yl)benzenesulfonamide; N1-(2-Thiazolyl)sulfanilamide; Benzenesulfonamide, 4-amino-N-2-thiazolyl-; 4-Amino-N-(1,3-Thiazol-2-Yl)Benzene-1-Sulfonamide; 4-amino-N-1,3-thiazol-2-ylbenzenesulfonamide; M&B 760; RP 2090; UNII-Y7FKS2XWQH; 4-Amino-N-thiazol-2-yl-benzenesulfonamide; 2090 R.P.; M+B 760; CHEBI:9337; Sulfanilamide, N1-2-thiazolyl-; Y7FKS2XWQH; Sodium sulfathiazole; CHEMBL437; N'-(2-Thiazolyl)sulfanilamide; N(sup1)-(2-Thiazolyl)sulfanilamide; MFCD00005319; NSC-31812; NSC683531; Sulfanilamide, N(sup1)-2-thiazolyl-; NSC-683531; CAS-72-14-0; NCGC00016309-02; NCGC00016309-06; Norsulfazolum; 4-Amino-N-(2-thiazolyl)benzenesulfonamide; DSSTox_CID_6068; DSSTox_RID_78004; DSSTox_GSID_26068; Solfatiazolo [DCIT]; Caswell No. 809B; Solfatiazolo; Sulfathiazol [INN-French]; Sulfatiazol [INN-Spanish]; N1-2-Thiazolylsulfanilamide; Sulfathiazolum [INN-Latin]; CCRIS 765; 2090 rp; 2-Sulfanilamidothiazol [German]; HSDB 4380; N(sup 1)-2-Thiazolylsulfanilamide; SR-05000001722; Sulfanilamide, N(1)-2-thiazolyl-; EINECS 200-771-5; NSC 31812; EPA Pesticide Chemical Code 077903; NSC 683531; Sulfanilamide, N(sup 1)-2-thiazolyl-; sulfthiazole; Enterobiocine; Sulfavitina; Cerazol; AI3-01050; Sulfathiazole [USP:INN:BAN]; 2-Sulfathiazole; YTZ; Prestwick_430; Sulfathiazole-13C6; Spectrum_001000; Prestwick0_000016; Prestwick1_000016; Prestwick2_000016; Prestwick3_000016; Spectrum2_000841; Spectrum3_001729; Spectrum4_000348; Spectrum5_001441; Sulfathiazole (USP/INN); N-2-Thiazolylsulfanilamide; Epitope ID:122234; Cambridge id 5251400; cid_5340; Oprea1_105970; Oprea1_297844; SCHEMBL94165; Triple sulfa (sulfathiazole); BSPBio_000051; BSPBio_003378; KBioGR_000755; KBioSS_001480; [(4-Aminophenyl)sulfonyl]-1,3-thiazol-2-ylamine; MLS002154174; N-1-2-Thiazolylsulfanilamide; DivK1c_000560; SPECTRUM1500553; Sulfathiazole-d4(benzene-d4); SPBio_000821; SPBio_001972; BPBio1_000057; WLN: T5N CSJ BMSWR DZ; DTXSID8026068; HMS501L22; KBio1_000560; KBio2_001480; KBio2_004048; KBio2_006616; KBio3_002598; N(sup1)-2-Thiazolylsulfanilamide; NINDS_000560; HMS1568C13; HMS1921C07; HMS2092K09; HMS2095C13; HMS2259A13; HMS3652A03; HMS3712C13; Pharmakon1600-01500553; ZINC121458; AMY33440; HY-B0507; NSC31812; SULFATHIAZOLE (TRIPLE SULFA); Tox21_110363; Tox21_202243; Tox21_303238; BDBM50027796; CCG-40296; NSC757331; s3116; STK043870; 2-(4-Aminobenzenesulfonamido)thiazole; AKOS000108630; Tox21_110363_1; DB06147; MCULE-1370710137; NSC-757331; SDCCGMLS-0065585.P001; IDI1_000560; NCGC00016309-01; NCGC00016309-03; NCGC00016309-04; NCGC00016309-05; NCGC00016309-07; NCGC00016309-08; NCGC00016309-09; NCGC00016309-10; NCGC00016309-14; NCGC00091133-01; NCGC00091133-02; NCGC00091133-03; NCGC00091133-04; NCGC00257187-01; NCGC00259792-01; Sulfanilamide, N1-2-thiazolyl- (8CI); AC-12783; DS-17245; K225; NCI60_002730; SMR000017368; Pyridine,2-(chloromethyl)-3,4-dimethoxy-; SBI-0051527.P004; Sulfanilamide, N1-4-thiazolin-2-ylidene-; DB-055610; 4-amino-N-(thiazol-2-yl)-benzenesulfonamide; AB00052102; BB 0245015; FT-0631310; Sulfathiazole 100 microg/mL in Acetonitrile; SW149625-4; 4-[(1,3-Thiazol-2-yl)aminosulfonyl]aniline; Sulfathiazole, analytical standard, >=98.0%; 9610-EP2295053A1; 9610-EP2308872A1; 9610-EP2316829A1; D01047; D70411; AB00052102_14; AB00052102_15; Q408427; Sulfathiazole, VETRANAL(TM), analytical standard; 4-Amino-N-(1,3-thiazol-2-yl)benzenesulfonamide #; Q-201765; SR-05000001722-1; SR-05000001722-3; Sulfathiazole, Antibiotic for Culture Media Use Only; BRD-K14705039-001-05-7; BRD-K14705039-001-08-1; F1443-4816; Sulfathiazole, European Pharmacopoeia (EP) Reference Standard; Sulfathiazole, United States Pharmacopeia (USP) Reference Standard
Click to Show/Hide
|
||||
| Structure |
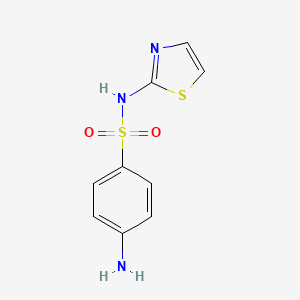
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C9H9N3O2S2
|
||||
| IsoSMILES |
C1=CC(=CC=C1N)S(=O)(=O)NC2=NC=CS2
|
||||
| InChI |
1S/C9H9N3O2S2/c10-7-1-3-8(4-2-7)16(13,14)12-9-11-5-6-15-9/h1-6H,10H2,(H,11,12)
|
||||
| InChIKey |
JNMRHUJNCSQMMB-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Dihydrofolate reductase (DHFR) | [1] | |||
| Resistant Disease | Escherichia coli infection [ICD-11: 1A03.0] | |||
| Molecule Alteration | Missense mutation | p.P64S |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Escherichia coli strain BN102 | 562 | ||
| Escherichia coli strain BN122 | 562 | |||
| Escherichia coli strain BN123 | 562 | |||
| Experiment for Molecule Alteration |
Direct PCR sequencing assay | |||
| Experiment for Drug Resistance |
MIC assay | |||
| Mechanism Description | Escherichia coli BN122 and BN123 folP sequences were identical to one another, but they contained a single difference from the wild-type nucleotide sequence. The difference was a C-to-T transition at nucleotide 184, resulting in a Pro-to-Ser substitution at amino acid 64. The sequence of this region of folP aligned with DHPS sequences from a variety of additional sources. Pro64 lies very close to the active site of DHPS, adjacent to Arg63, whose side chain bonds in a hydrogen bond with an oxygen of the sulfanilamide inhibitor. Substitution of Pro64 by Ser is likely to alter the local structure of the peptide, in turn altering the ability of Arg63 to contact the inhibitor. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
