Drug Information
Drug (ID: DG00387) and It's Reported Resistant Information
| Name |
Methymycin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Methymycin; UNII-16QGD97DXG; 497-72-3; 16QGD97DXG; CHEBI:29630; (3r,4s,5s,7r,9e,11s,12r)-12-Ethyl-11-Hydroxy-3,5,7,11-Tetramethyl-2,8-Dioxooxacyclododec-9-En-4-Yl 3,4,6-Trideoxy-3-(Dimethylamino)-Beta-D-Xylo-Hexopyranoside; (3R,4S,5S,7R,9E,11S,12R)-4-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-12-ethyl-11-hydroxy-3,5,7,11-tetramethyl-1-oxacyclododec-9-ene-2,8-dione; MT9; SCHEMBL654455; CHEMBL489347; LMPK04000037; Q27110190
Click to Show/Hide
|
||||
| Structure |
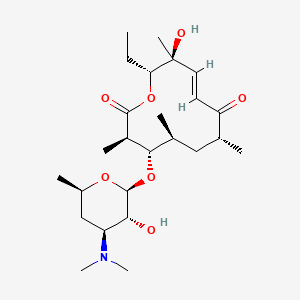
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C25H43NO7
|
||||
| IsoSMILES |
CC[C@@H]1[C@@](/C=C/C(=O)[C@@H](C[C@@H]([C@@H]([C@H](C(=O)O1)C)O[C@H]2[C@@H]([C@H](C[C@H](O2)C)N(C)C)O)C)C)(C)O
|
||||
| InChI |
1S/C25H43NO7/c1-9-20-25(6,30)11-10-19(27)14(2)12-15(3)22(17(5)23(29)32-20)33-24-21(28)18(26(7)8)13-16(4)31-24/h10-11,14-18,20-22,24,28,30H,9,12-13H2,1-8H3/b11-10+/t14-,15+,16-,17-,18+,20-,21-,22+,24+,25+/m1/s1
|
||||
| InChIKey |
HUKYPYXOBINMND-HYUJHOPRSA-N
|
||||
| PubChem CID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: srmA open reading frame gimA (GIMA) | [1] | |||
| Resistant Disease | Streptomyces ambbyaciens infection [ICD-11: 1C43.0] | |||
| Molecule Alteration | Expression | Inherence |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Escherichia coli | 668369 | ||
| Escherichia coli strain S17.1 | 1227813 | |||
| Micrococcus luteus strain Cgr | 1270 | |||
| Micrococcus luteus strain DSM1790 | 1270 | |||
| Streptomyces ambofaciens strain ATCC 23877 | 278992 | |||
| Streptomyces ambofaciens strain OS41.99 | 1954 | |||
| Streptomyces ambofaciens strain OS41.99NP | 1954 | |||
| Streptomyces ambofaciens strain OS81 | 1954 | |||
| Streptomyces lividans strain OS456 | 1916 | |||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Experiment for Drug Resistance |
Observation of growth inhibition zones assay | |||
| Mechanism Description | With UDP-[14C]glucose as the cofactor, crude S30 extracts from OS456(pOS41.90) were tested on various macrolides. Among those, chalcomycin was the most active substrate. Methymycin, tylosin, pikromycin, and rosaramicin were four of the best substrates. Oleandomycin, josamycin, and carbomycin were glycosylated to a lesser extent. Macrolides that were found to be as poor substrates of GimA as lankamycin were erythromycin and angolamycin. Spiramycin was also a very poor substrate. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
