Drug Information
Drug (ID: DG00362) and It's Reported Resistant Information
| Name |
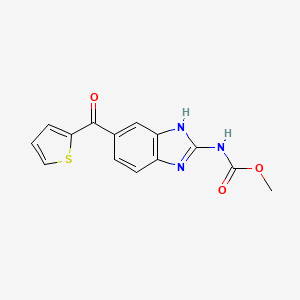
Nocodazole
|
||||
|---|---|---|---|---|---|
| Synonyms |
Nocodazole; 31430-18-9; Oncodazole; R 17934; Nocodazolum; Nocodazol; NSC 238159; R 17,934; Methyl N-[6-(thiophene-2-carbonyl)-1H-benzimidazol-2-yl]carbamate; C14H11N3O3S; methyl (5-(thiophene-2-carbonyl)-1H-benzo[d]imidazol-2-yl)carbamate; UNII-SH1WY3R615; Methyl 5-(2-thenoyl)-2-benzimidazolecarbamate; Methyl N-(5-thenoyl-2-benzimidazolyl)carbamate; NSC-238159; Methyl [5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl]carbamate; N-(5-(2-Thenoyl)-2-benzimidazolyl)carbamic acid methyl ester; NSC238159; Methyl (5-(2-thienylcarbonyl))-1H-benzimidazole-2-ylcarbamate; R-17934; 2-Benzimidazolecarbamic acid, 5-(2-thienylcarbonyl)-, methyl ester; CHEMBL9514; MLS001164242; SH1WY3R615; 2-Benzimidazolecarbamic acid, 5-(2-thenoyl)-, methyl ester; CHEBI:34892; Methyl (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl)carbamate; Carbamic acid, (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl)-, methyl ester; MFCD00005588; NCGC00015647-05; Nocidazole; SMR000326904; CAS-31430-18-9; [5-(2-Thienylcarbonyl)-1H-benzimidazol-2-yl]-carbamic acid methyl ester; methyl (6-(thiophene-2-carbonyl)-1H-benzo[d]imidazol-2-yl)carbamate; Carbamic acid, (5-(2-thienylcarbonyl)-1H-benzimidazole-2-yl)-, methyl ester; Carbamic acid, [5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl]-, methyl ester; Methyl 5-(2-thienoyl)-2-benzimidazolecarbamate; DSSTox_CID_11800; DSSTox_RID_78890; DSSTox_GSID_31800; 2-Benzimidazolecarbamic acid, 5-(2-thienoyl)-, methyl ester; Methyl [5-(2-thienylcarbonyl)-1H- benzimidazol-2-yl]carbamate; methyl [6-(thiophen-2-ylcarbonyl)-1H-benzimidazol-2-yl]carbamate; N-(5-(2-Thienoyl)-2-benzimidazolyl)carbamic acid methyl ester; methyl N-[5-(thiophene-2-carbonyl)-1H-benzimidazol-2-yl]carbamate; Nocodazole [USAN:INN]; Nocodazol [INN-Spanish]; Nocodazolum [INN-Latin]; R17934; SR-01000075979; EINECS 250-626-5; NSC 238 159; Nococazole; Methyl(5-(2-thienylcarbonyl))-1H-benzimidazole-2-yl carbamate; Methyl[5-(2-thienylcarbonyl)]-1H-benzimidazole-2-yl carbamate; N-[5-(2-Thenoyl)-2-benzimidazolyl]carbamic acid methyl ester; N-[5-(2-Thienoyl)-2-benzimidazolyl]carbamic acid methyl ester; (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl)-carbamic acid methyl ester; Carbamic acid, (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl)-, methyl ester (9CI); Carbamic acid, [5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl]-, methyl ester (9CI); NZO; Prestwick_359; Tocris-1228; 3ee2; Nocodazole (USAN/INN); Prestwick0_000100; Prestwick1_000100; Prestwick2_000100; Prestwick3_000100; Spectrum3_001768; Spectrum4_001060; Lopac-M-1404; ChemDiv1_000089; Probes1_000012; Probes1_000176; Probes2_000086; Probes2_000148; UPCMLD-DP111; Cambridge id 5175348; cid_4122; SCHEMBL9477; Lopac0_000733; Oprea1_483899; Oprea1_741554; BSPBio_000060; BSPBio_000982; BSPBio_003235; CBDivE_004971; CBDivE_008428; KBioGR_000322; KBioGR_001360; KBioSS_000322; MLS000860046; BIDD:GT0494; SPECTRUM1503266; Nocodazole Ready Made Solution; SPBio_001999; BPBio1_000066; DTXSID9031800; UPCMLD-DP111:001; BCBcMAP01_000161; BDBM97233; HMS587E01; KBio2_000322; KBio2_002890; KBio2_005458; KBio3_000643; KBio3_000644; KBio3_002735; ZINC56509; AOB6196; Bio1_000461; Bio1_000950; Bio1_001439; Bio2_000331; Bio2_000811; HMS1362B03; HMS1568C22; HMS1792B03; HMS1922O09; HMS1990B03; HMS2094G13; HMS2095C22; HMS2235E18; HMS3262C08; HMS3267F03; HMS3403B03; HMS3412A10; HMS3604E13; HMS3656J06; HMS3676A10; HMS3712C22; Pharmakon1600-01503266; BCP07558; EX-A5289; Tox21_110189; Tox21_500733; 2593AH; NSC759882; s2775; STK832483; Nocodazole, >=99% (TLC), powder; AKOS000538825; AKOS015901529; Tox21_110189_1; API0003615; CCG-101240; CCG-208075; CP-0076; CS-1893; DB08313; LP00733; MCULE-3133043736; NSC-759882; SB19455; SDCCGSBI-0050711.P003; IDI1_002086; QTL1_000062; SMP2_000261; NCGC00015647-01; NCGC00015647-02; NCGC00015647-03; NCGC00015647-04; NCGC00015647-06; NCGC00015647-07; NCGC00015647-08; NCGC00015647-09; NCGC00015647-10; NCGC00015647-11; NCGC00015647-12; NCGC00015647-13; NCGC00015647-14; NCGC00015647-15; NCGC00015647-18; NCGC00015647-33; NCGC00025058-01; NCGC00025058-02; NCGC00025058-03; NCGC00025058-04; NCGC00025058-05; NCGC00025058-06; NCGC00025058-07; NCGC00025058-08; NCGC00025058-09; NCGC00025058-10; NCGC00261418-01; AC-25615; AM807900; HY-13520; NCI60_001911; SBI-0050711.P002; AB0109635; EU-0100733; FT-0660415; SW102861-5; D05197; M 1404; 4-HYDROXY-3-IODO-BIPHENYL-4-CARBONITRILE; R17,934; Q2506092; SR-01000075979-1; SR-01000075979-3; SR-01000075979-6; SR-01000075979-9; W-202288; BRD-K12539581-001-06-2; BRD-K12539581-001-14-6; methyl 6-(thien-2-ylcarbonyl)-1H-benzimidazol-2-ylcarbamate; 2-Benzimidazolecarbamic acid, 5-(2-thenoyl)-, methyl ester (8CI); methyl [5-(thiophen-2-ylcarbonyl)-1H-benzimidazol-2-yl]carbamate; methyl N-(6-thiophen-2-ylcarbonyl-1H-benzimidazol-2-yl)carbamate; methyl(6-(thiophene-2-carbonyl)-1H-benzo[d]imidazol-2-yl)carbamate; N-[6-(2-thenoyl)-1H-benzimidazol-2-yl]carbamic acid methyl ester; [[5-(2-Thienylcarbonyl)-1H-benzimidazol-2-yl]]carbamic acid methyl ester; [5-(2-Thienylcarbonyl)-1H-benzimidazol-2-yl]carbonic acid, methyl ester; carbamic acid, [6-(2-thienylcarbonyl)-1H-benzimidazol-2-yl]-, methyl ester; Carbamic acid, N-[5-(2-thenoyl)-1H-benzimidazol-2-yl]-, methyl ester; [5-(Thiophene-2-carbonyl)-1H-benzoimidazol-2-yl]-carbamic acid methyl ester; Carbamic acid, N-[6-(2-thienylcarbonyl)-1H-benzimidazol-2-yl]-, methyl ester; N-[6-[oxo(thiophen-2-yl)methyl]-1H-benzimidazol-2-yl]carbamic acid methyl ester
Click to Show/Hide
|
||||
| Structure |
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C14H11N3O3S
|
||||
| IsoSMILES |
COC(=O)NC1=NC2=C(N1)C=C(C=C2)C(=O)C3=CC=CS3
|
||||
| InChI |
1S/C14H11N3O3S/c1-20-14(19)17-13-15-9-5-4-8(7-10(9)16-13)12(18)11-3-2-6-21-11/h2-7H,1H3,(H2,15,16,17,19)
|
||||
| InChIKey |
KYRVNWMVYQXFEU-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Pleiotropic ABC efflux transporter of multiple drugs CDR1 (CDR1) | [1] | |||
| Sensitive Disease | Recurrent oropharyngeal candidiasis [ICD-11: 1F23.6] | |||
| Molecule Alteration | Deletion mutation | Deleteion |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Candida albicans strain DSY448 | 5476 | ||
| Experiment for Molecule Alteration |
PCR; Southern blotting analysis; Northern blottling analysis | |||
| Experiment for Drug Resistance |
Growth differences between the different C. albicans strains assay | |||
| Mechanism Description | The delta cdr1 mutant was slightly more susceptible than the wild type to nocodazole, cerulenin, and crystal violet but not to amphotericin B, nikkomy- cin Z, flucytosine, or pradimicin. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
