Drug Information
Drug (ID: DG00319) and It's Reported Resistant Information
| Name |
Sitafloxacin
|
||||
|---|---|---|---|---|---|
| Synonyms |
127254-12-0; Gracevit; Sitafloxacin Sesquihydrate; DU 6859; 163253-35-8; UNII-3GJC60U4Q8; DU-6859a; Sitafloxacin isomer II; DU 6859A; C19H18ClF2N3O3; 3GJC60U4Q8; CHEBI:4304; 7-[(7S)-7-amino-5-azaspiro[2.4]heptan-5-yl]-8-chloro-6-fluoro-1-[(1R,2S)-2-fluorocyclopropyl]-4-oxoquinoline-3-carboxylic acid; Sitafloxacin [INN]; 7-((S)-7-AMINO-5-AZASPIRO[2.4]HEPTAN-5-YL)-8-CHLORO-6-FLUORO-1-((1R,2S)-2-FLUOROCYCLOPROPYL)-4-OXO-1,4-DIHYDROQUINOLINE-3-CARBOXYLIC ACID; STFX; DU-6859; 127254-10-8; Sitafloxacin isomer III (RRS); 7-[(7S)-7-amino-5-azaspiro[2.4]heptan-5-yl]-8-chloro-6-fluoro-1-[(1R,2S)-2-fluorocyclopropyl]-4-oxo-3-quinolinecarboxylic acid; 7-[(7S)-7-azanyl-5-azaspiro[2.4]heptan-5-yl]-8-chloranyl-6-fluoranyl-1-[(1R,2S)-2-fluoranylcyclopropyl]-4-oxidanylidene-quinoline-3-carboxylic acid; SITAFLOXACIN ISOMER III(RRS); SITAFLOXACINISOMER; SCHEMBL74553; 7-((7S)-Amino-5-azaspiro(2,4)heptan-5-yl)-8-chloro-6-fluoro-1-((1R,2R)-cis-2-fluoro-1-cyclopropyl)-1,4-dihydro-4-oxoquinolone-3-carboxylic acid; 7-(7-Amino-5-azaspiro(2.4)heptan-5-yl)-8-chloro-6-fluoro-1-(2-fluorocyclopropyl)-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid sesquihydrate; SITAFLOXACINISOMER(RRS); SITAFLOXACINISOMER(RSR); DU6859a; CHEMBL108821; AMSP00027; GTPL11040; HY-B0395; ZINC3795983; AKOS015962212; AC-1388; ACN-048224; AM85541; compound 33 [PMID: 7932562]; YF10030; (-)-7-((7S)-Amino-5-azaspiro(2,4)hept-5-yl)-8-chloro-6-fluoro-1-((1R,2S)-2-fluorocyclopropyl)-1,4-dihydro-4-oxoq-3-uinolonecarboxylic acid; 3-Quinolinecarboxylic acid, 7-(7-amino-5-azaspiro(2.4)hept-5-yl)-8-chloro-6-fluoro-1-(2-fluorocyclopropyl)-1,4-dihydro-4-oxo-, (1R-(1alpha(S*),2alpha))-; 3-Quinolinecarboxylic acid, 7-[(7S)-7-amino-5-azaspiro[2.4]hept-5-yl]-8-chloro-6-fluoro-1-[(1R,2S)-2-fluorocyclopropyl]-1,4-dihydro-4-oxo-; 7-[(7S)-7-amino-5-azaspiro[2.4]heptan-5-yl]-8-chloro-6-fluoro-1-[(1R,2S)-2-fluorocyclopropyl]-4-oxo-quinoline-3-carboxylic acid; AT-21032; AB01568244_01; 253S358; A805671; A805673; J-519022; (-)-7-[(7s)-7-amino-5-azaspiro[2.4]heptan-5-yl]-8-chloro-6-fluoro-1-[(1r,2s)-2-fluoro-1-cyclopropyl]-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid; 273401-03-9; 3-Quinolinecarboxylic acid, 7-((7S)-7-amino-5-azaspiro(24)hept-5-yl)-8-chloro-6-fluoro-1-((1R,2S)-2-fluorocyclopropyl)-1,4-dihydro-4-oxo-; 7-((7S)-7-Amino-5-azaspiro[2.4]hept-5-yl)-1-((1S,2S)-2-fluorocyclopropyl)-8-chloro-6-fluoro-4-oxohydroquinoline-3-carboxylic acid; 7-((S)-7-Amino-5-azaspiro[2.4]Heptan-5-yl)-8-chloro-6-fluoro-1-((1R,2S)-2-fluorocyclopropyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; 7-[(7S)-7-Amino-5-azaspiro[2.4]hept-5-yl]-8-chloro-6-fluoro-1-[(1 R,2S)-2-fluorocyclopropyl]-4-oxo-1,4-dihydro-3-quinolinecarboxyli c acid; rel-7-((R)-7-Amino-5-azaspiro[2.4]heptan-5-yl)-8-chloro-6-fluoro-1-((1S,2R)-2-fluorocyclopropyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
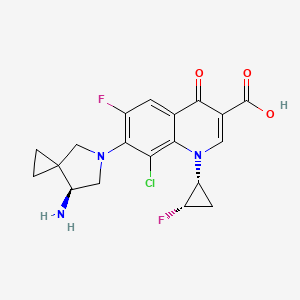
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Bacterial DNA gyrase (Bact gyrase) |
GYRA_STAAU
; GYRB_STAAU |
[1] | ||
| Bacterial DNA topoisomerase 4A (Bact parC) | PARC_ECOLI | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C19H18ClF2N3O3
|
||||
| IsoSMILES |
C1CC12CN(C[C@H]2N)C3=C(C=C4C(=C3Cl)N(C=C(C4=O)C(=O)O)[C@@H]5C[C@@H]5F)F
|
||||
| InChI |
1S/C19H18ClF2N3O3/c20-14-15-8(17(26)9(18(27)28)5-25(15)12-4-10(12)21)3-11(22)16(14)24-6-13(23)19(7-24)1-2-19/h3,5,10,12-13H,1-2,4,6-7,23H2,(H,27,28)/t10-,12+,13+/m0/s1
|
||||
| InChIKey |
PNUZDKCDAWUEGK-CYZMBNFOSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: DNA topoisomerase 4 subunit B (PARE) | [1] | |||
| Resistant Disease | Leprosy [ICD-11: 1B20.0] | |||
| Molecule Alteration | Missense mutation | p.D464N |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli BL21 (DE3) | 469008 | ||
| Escherichia coli Rosetta-gami 2 | 562 | |||
| Escherichia coli TOP-10 | 83333 | |||
| Mycobacterium leprae Thai-53 | 1769 | |||
| Experiment for Molecule Alteration |
Whole genome sequence assay; Allelic frequency measurement assay | |||
| Experiment for Drug Resistance |
DNA supercoiling assay; DNA cleavage assay | |||
| Mechanism Description | FQs are known to interact with both A and B subunits of DNA gyrase and inhibit supercoiling activity of this enzyme.The FQ-inhibited supercoiling assay and FQ-induced cleavage assay demonstrated the important roles of these amino acid substitutions in reduced sensitivity to FQ with marked influence by amino acid substitution, especially at position 502. | |||
| Key Molecule: DNA topoisomerase 4 subunit B (PARE) | [1] | |||
| Resistant Disease | Leprosy [ICD-11: 1B20.0] | |||
| Molecule Alteration | Missense mutation | p.N502D |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli BL21 (DE3) | 469008 | ||
| Escherichia coli Rosetta-gami 2 | 562 | |||
| Escherichia coli TOP-10 | 83333 | |||
| Mycobacterium leprae Thai-53 | 1769 | |||
| Experiment for Molecule Alteration |
Whole genome sequence assay; Allelic frequency measurement assay | |||
| Experiment for Drug Resistance |
DNA supercoiling assay; DNA cleavage assay | |||
| Mechanism Description | FQs are known to interact with both A and B subunits of DNA gyrase and inhibit supercoiling activity of this enzyme.The FQ-inhibited supercoiling assay and FQ-induced cleavage assay demonstrated the important roles of these amino acid substitutions in reduced sensitivity to FQ with marked influence by amino acid substitution, especially at position 502. | |||
| Key Molecule: DNA topoisomerase 4 subunit B (PARE) | [1] | |||
| Resistant Disease | Leprosy [ICD-11: 1B20.0] | |||
| Molecule Alteration | Missense mutation | p.E504V |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli BL21 (DE3) | 469008 | ||
| Escherichia coli Rosetta-gami 2 | 562 | |||
| Escherichia coli TOP-10 | 83333 | |||
| Mycobacterium leprae Thai-53 | 1769 | |||
| Experiment for Molecule Alteration |
Whole genome sequence assay; Allelic frequency measurement assay | |||
| Experiment for Drug Resistance |
DNA supercoiling assay; DNA cleavage assay | |||
| Mechanism Description | FQs are known to interact with both A and B subunits of DNA gyrase and inhibit supercoiling activity of this enzyme.The FQ-inhibited supercoiling assay and FQ-induced cleavage assay demonstrated the important roles of these amino acid substitutions in reduced sensitivity to FQ with marked influence by amino acid substitution, especially at position 502. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
