Drug Information
Drug (ID: DG00299) and It's Reported Resistant Information
| Name |
Cefuroxime
|
||||
|---|---|---|---|---|---|
| Synonyms |
Anaptivan; Biociclin; Biofuroksym; Bioxima; CXM; Cefofix; Cefumax; Cefurex; Cefuril; Cefuroxim; Cefuroximesodium; Cefuroximine; Cefuroximo; Cefuroximum; Cephuroxime; Cetroxil; Colifossim; Curocef; Curoxim; Curoxima; Curoxime; Froxal; Furoxil; Kesint; Ketocef; Lifurox; Medoxim; Sharox; Spectrazolr; Ultroxim; Zinacef;CEFUROXIME AND DEXTROSE IN DUPLEX CONTAINER; CEFUROXIME SODIUM; Cefuroxim AJ; Cefuroxim Fresenius; Cefuroxim Genericsn; Cefuroxim Hexal; Cefuroxim Lilly; Cefuroxim MN; Cefuroxim Norcox; Cefuroxim curasan; Cefuroxima Fabra; Cefuroxima Richet; Cefuroxime for Injection and Dextrose for Injection in Duplex Container; Cefuroxime na; Cefuroxime sodium salt; KEFUROX IN PLASTIC CONTAINER; Sodium cefuroxime; ZINACEF IN PLASTIC CONTAINER; Zinacef Danmark; Ceftin (TN); Cefuroxim Norcox [inj.]; Cefuroxime (TN); Cefuroximo [INN-Spanish]; Cefuroximum [INN-Latin]; Cetroxil [inj.]; Froxal [inj.]; KS-1040; Sharox [inj.]; Zinacef (TN); Zinnat (TN); Zinnat [inj.]; Cefuroxime (USAN/INN); Cefuroxime [USAN:INN:BAN]; Cefuroxime sodium (JP15/USP); Cefuroxime sodium [USAN:BAN:JAN]; Sodium (6R-(6alpha,7beta(Z)))-3-(((aminocarbonyl)oxy)methyl)-7-(2-furyl(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate; Sodium (6R,7R)-7-(2-(2-furyl)glyoxylamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate, 7(sup 2)-(Z)-(O-methyloxime), carbamate (ester); (6R,7R)-3-(carbamoyloxymethyl)-7-[[(2Z)-2-(furan-2-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-[(carbamoyloxy)methyl]-7-[(2Z)-2-(furan-2-yl)-2-(methoxyimino)acetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-[(carbamoyloxy)methyl]-7-{[(2Z)-2-furan-2-yl-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-(2-(2-Furyl)glyoxylamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid 7(sup 2)-(Z)-(O-methyloxime) carbamate (ester); 3-[(carbamoyloxy)methyl]-7beta-[(2Z)-2-(furan-2-yl)-2-(methoxyimino)acetamido]-3,4-didehydrocepham-4-carboxylic acid
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
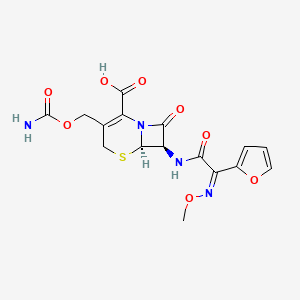
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
|
||||
| Target | Bacterial Penicillin binding protein (Bact PBP) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C16H16N4O8S
|
||||
| IsoSMILES |
CO/N=C(/C1=CC=CO1)\\C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)COC(=O)N)C(=O)O
|
||||
| InChI |
1S/C16H16N4O8S/c1-26-19-9(8-3-2-4-27-8)12(21)18-10-13(22)20-11(15(23)24)7(5-28-16(17)25)6-29-14(10)20/h2-4,10,14H,5-6H2,1H3,(H2,17,25)(H,18,21)(H,23,24)/b19-9-/t10-,14-/m1/s1
|
||||
| InChIKey |
JFPVXVDWJQMJEE-IZRZKJBUSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Beta-lactamase (BLA) | [1] | |||
| Resistant Disease | Bacterial infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Missense mutation | p.Y221H |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli TOP10 | 83333 | ||
| Escherichia coli EC13 | 562 | |||
| Experiment for Molecule Alteration |
Whole genome sequencing assay | |||
| Experiment for Drug Resistance |
Disk diffusion test assay | |||
| Mechanism Description | The CMY-136 Beta-lactamase, a Y221H point mutant derivative of CMY-2,confers an increased level of resistance to ticarcillin, cefuroxime, cefotaxime, and ceftolozane/tazobactam. | |||
| Key Molecule: Beta-lactamase (BLA) | [4] | |||
| Resistant Disease | Pseudomonas aeruginosa infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Expression | Inherence |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Pseudomonas aeruginosa PAO1 | 208964 | ||
| Experiment for Molecule Alteration |
DNA sequencing and protein assay | |||
| Experiment for Drug Resistance |
Disk diffusion assay | |||
| Mechanism Description | P. aeruginosa harbors two naturally encoded Beta-lactamase genes, one of which encodes an inducible cephalosporinase and the other of which encodes a constitutively expressed oxacillinase. AmpC is a kind of cephalosporinase which lead to drug resistance. | |||
| Key Molecule: Beta-lactamase (BLA) | [2], [3] | |||
| Resistant Disease | Bacterial infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Missense mutation | p.D240G |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli | 668369 | ||
| Escherichia coli Gre-1 | 562 | |||
| Experiment for Molecule Alteration |
Whole genome sequence assay | |||
| Experiment for Drug Resistance |
Agar dilution method assay | |||
| Mechanism Description | The first extended-spectrum Beta-lactamase (ESBL) of the CTX-M type (MEN-1/CTX-M-1) was reported at the beginning of the 1990s.CTX-M-27 differed from CTX-M-14 only by the substitution D240G and was the third CTX-M enzyme harbouring this mutation after CTX-M-15 and CTX-M-16. The Gly-240-harbouring enzyme CTX-M-27 conferred to Escherichia coli higher MICs of ceftazidime (MIC, 8 versus 1 mg/L) than did the Asp-240-harbouring CTX-M-14 enzyme. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
