Drug Information
Drug (ID: DG00067) and It's Reported Resistant Information
| Name |
Ceftibuten
|
||||
|---|---|---|---|---|---|
| Synonyms |
CETB; Cedax; Ceftem; Ceftibutene; Ceftibuteno; Ceftibutenum; Cephem; Ceprifran; Isocef; Keimax; Antibiotic 7432S; S 7432; Sch 39720; Cedax (TN); Ceftibutene [INN-French]; Ceftibuteno [INN-Spanish]; Ceftibutenum [INN-Latin]; Cephalosporin 7432-S; Cis-Ceftibutin; Cis-ceftibuten; Sch-39720; Trans-Ceftibuten; Ceftibuten(USAN/INN); Ceftibuten [USAN:INN:BAN]; (+)-(6R,7R)-7-((Z)-2-(2-Amino-4-thiazolyl)-4-carboxycrotonamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(E)-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(Z)-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-[[(E)-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-[[(Z)-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7-[[2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 7432-S; 7beta-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino}-3,4-didehydrocepham-4-carboxylic acid
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
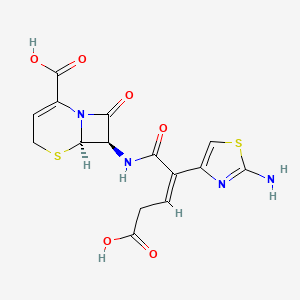
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
|
||||
| Target | Bacterial Penicillin binding protein (Bact PBP) | NOUNIPROTAC | [2] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C15H14N4O6S2
|
||||
| IsoSMILES |
C1C=C(N2[C@H](S1)[C@@H](C2=O)NC(=O)/C(=C\\CC(=O)O)/C3=CSC(=N3)N)C(=O)O
|
||||
| InChI |
1S/C15H14N4O6S2/c16-15-17-7(5-27-15)6(1-2-9(20)21)11(22)18-10-12(23)19-8(14(24)25)3-4-26-13(10)19/h1,3,5,10,13H,2,4H2,(H2,16,17)(H,18,22)(H,20,21)(H,24,25)/b6-1-/t10-,13-/m1/s1
|
||||
| InChIKey |
UNJFKXSSGBWRBZ-BJCIPQKHSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Beta-lactamase (BLA) | [1], [2] | |||
| Resistant Disease | Bacterial infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli strain DH5a | 668369 | ||
| Klebsiella pneumoniae strain HEL-1 | 573 | |||
| Experiment for Molecule Alteration |
Whole genome sequence assay | |||
| Experiment for Drug Resistance |
Agar dilution method assay | |||
| Mechanism Description | The phenotype of Klebsiella pneumoniae HEL-1 indicates a plasmidic cephamycinase gene (blaCMY-2),which is responsible for cephamycin resistance. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
