Drug Information
Drug (ID: DG00036) and It's Reported Resistant Information
| Name |
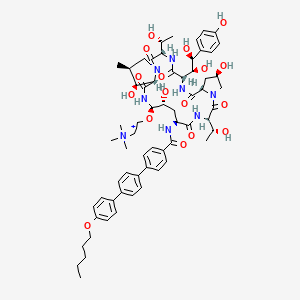
Rezafungin
|
||||
|---|---|---|---|---|---|
| Synonyms |
CD-101; CD101; SP-3025; CHEMBL3989945; HY-108009; CS-0027142; J3.555.717B; J3.599.425D; (4R,5R)-N2-[4-(4'-Pentoxy-1,1'-biphenyl-4-yl)benzoyl]-5-[2-(trimethylaminio)ethoxy]-4-hydroxy-cyclo[L-Orn*-[(3R)-3-methyl-L-Ser-]-[(4R)-4-hydroxy-L-Pro-]-2-[(1S,2S)-1,2-dihydroxy-2-(4-hydroxyphenyl)ethyl]-L-Gly-[(3R)-3-methyl-L-Ser-]-[(3S,4S)-3-hydroxy-4-methyl-L-Pro-]-]
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Fungal 1,3-beta-glucan synthase (Fung GSC2) | FKS2_YEAST | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C63H85N8O17+
|
||||
| IsoSMILES |
CCCCCOC1=CC=C(C=C1)C2=CC=C(C=C2)C3=CC=C(C=C3)C(=O)N[C@H]4C[C@H]([C@H](NC(=O)[C@@H]5[C@H]([C@H](CN5C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H]6C[C@H](CN6C(=O)[C@@H](NC4=O)[C@@H](C)O)O)[C@@H]([C@H](C7=CC=C(C=C7)O)O)O)[C@@H](C)O)C)O)OCC[N+](C)(C)C)O
|
||||
| InChI |
1S/C63H84N8O17/c1-8-9-10-28-87-45-25-21-40(22-26-45)38-13-11-37(12-14-38)39-15-17-42(18-16-39)56(80)64-46-31-48(76)61(88-29-27-71(5,6)7)68-60(84)52-53(77)34(2)32-70(52)63(86)50(36(4)73)66-59(83)51(55(79)54(78)41-19-23-43(74)24-20-41)67-58(82)47-30-44(75)33-69(47)62(85)49(35(3)72)65-57(46)81/h11-26,34-36,44,46-55,61,72-73,75-79H,8-10,27-33H2,1-7H3,(H5-,64,65,66,67,68,74,80,81,82,83,84)/p+1/t34-,35+,36+,44+,46-,47-,48+,49-,50-,51-,52-,53-,54-,55-,61+/m0/s1
|
||||
| InChIKey |
LNFCWEXGZIEGJW-TXSVMFMRSA-O
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: D-glucan-1,3-beta--UDP glucosyltransferase (FKS1) | [1] | |||
| Resistant Disease | Candida auris infection [ICD-11: 1F23.2] | |||
| Molecule Alteration | Missense mutation | p.S639P |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Candida auris strain | 498019 | ||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Experiment for Drug Resistance |
Broth microdilution assay | |||
| Mechanism Description | Sequencing of FkS revealed that 4 isolates contain the amino acid substitution S639P and those isolates exhibit the highest MICs to echinocandins (micafungin, caspofungin, and anidulafungin, CD101). | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
